��Ŀ����
ij�о���ѧϰС������ͭм������ͭ������������������ɵĻ��ᷴӦ����ȡCuSO4��5H2O���壬����������Ļ�ԭ����ΪNO����Ӧ�����в�����SO2,��Ӧ�����Һ�в���Cu(NO3)2����Ӧ�й�����ȫ�ܽ⣬�������ǡ����ȫ��Ӧ������������������Ϊ480 g������ͭ����������Ϊx���Իش����С�
��1����������ͭ���������yΪ�� g����x��ʾ����
��2����x =0.4,������HNO3��H2SO4�����ʵ���֮��Ϊ�� ��
��3����x=0.4,480g����������һ���������Ⱥ�ַ�Ӧ����ȴǡ��ֻ�õ�CuSO4��5H2O,����ԭ������H2SO4������������
��1��375x + 1500 ��2��1��3.3
(3)n(Cu)= 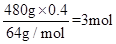
n(CuO)= 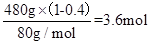
m(H2SO4)=(3mol+3.6mol)��98g/mol=646.8g
m(NO)="2/3" n(Cu)��30g/mol=60g
��(H2SO4)= 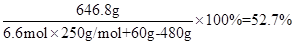
�������52%-53%���۷֣�������֣�
����

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ
 ��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�
��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ� ��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3��
��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3��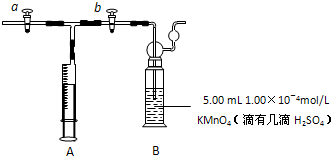
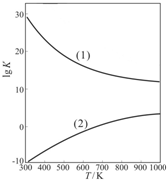
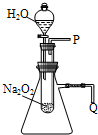 ����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������
����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������