��Ŀ����
 ��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3��
��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3����1������˵������������������
a
a
��������ĸ��a���ü�ȩ��Һ����ˮ��Ʒ�Գ�ʱ�䱣��ˮ��Ʒ�ġ����ʡ�
b����װ���·���סǰ�����ڱ���һ���¶Ȳ�ע��ͨ��
c���ϳɡ����顱�ķ�ӦNaHSO3+HCHO��NaO-CH2-SO3H�Ǽӳɷ�Ӧ
d���������ֿ�������������걾����ʬ�壩
��2��ij�о���ѧϰС�����ü�ȩ���ⶨ�����̬���ʵĺ�����
�����ϣ�4NH4++6HCHO=��CH2��6N4H++3H++6H2O�������ɵ�H+�ͣ�CH2��6N4H+����NaOH����Һ�ζ������÷�̪��ָʾ������
�ü�ȩ���ⶨ�����������ʺϵ������
a
a
��������ĸ��a��NH4HCO3 b����NH4��2SO4 c��NH4Cl
��3����ҵ�Ƽ�ȩ�����ַ������£����ݾ�Ϊ298.15K�²ⶨ����
��ӦI��
CH3OH��g����HCHO��g��+H2��g����H1=+92.09kJ/mol��K1=3.92��10-11
��ӦII��
CH3OH��g��+
| 1 | 2 |
����ɫ��ѧ�ᳫ��������Ӧ���ԭ�������ʣ���Ӧ
I
I
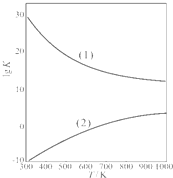
���I����II�����Ƽ�ȩԭ�������ʸ��ߣ��ӷ�Ӧ���ʱ��ƽ�ⳣ��Kֵ������ӦII
II
���I����II�����Ƽ�ȩ�����������ڷ�Ӧ���Է����е�������
C
C
������ĸ����a������b������c���κ�����
������ͼ�Ǽ״��Ƽ�ȩ�йط�Ӧ��lgK��ƽ�ⳣ���Ķ���ֵ���¶�T�ı仯��ͼ�����ߣ�1����ʾ���Ƿ�Ӧ
II
II
���I����II��������4��ij�о���ѧϰС��ⶨ�����м�ȩ�ĺ�����ԭ�����£�
4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O
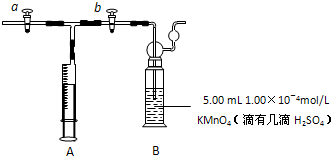
��a���ر�b����ע������ȡ���ڿ������ر�a����b�����ƶ�ע�����������建����������KMnO4��Һ�У�ʹ���ַ�Ӧ����Bװ����
����KMnO4��Һ��ɫͻȻ��ȥ
����KMnO4��Һ��ɫͻȻ��ȥ
ʱֹͣ���������ܹ�ȡ��Ϊ50L��������þ����ڿ����м�ȩ��Ũ��0.375
0.375
mg/m3����������1��a����ȩ�ж���
b����װ�����к��д����ļ�ȩ�������к����ʵĽǶȷ�����
c�����ݼӳɷ�Ӧ�ĸ��
d�����ݼ�ȩ���ƻ������ʵĽṹ���ʿ����жϣ�
��2������HCO3-Ҳ����NaOH����Һ��Ӧ��
��3���ٸ���ԭ�������ʱ�ʾĿ������������������������֮�����Ƚϣ�����ƽ�ⳣ��Խ��Խ�����ڷ�Ӧ�Ľ��У�
�ڷ�Ӧ���ȣ�����ֵ�����ݡ�G=��H-T?��S�жϣ�
�۸����¶ȶ�ƽ�ⳣ����Ӱ�죻
��4������ǡ�÷�Ӧ�����꣬����KMnO4��Һ��ɫ��ȥ�����ݻ�ѧ����ʽ4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O���HCHO�����ʵ�����Ȼ��������������������ȩ��Ũ�ȣ�
b����װ�����к��д����ļ�ȩ�������к����ʵĽǶȷ�����
c�����ݼӳɷ�Ӧ�ĸ��
d�����ݼ�ȩ���ƻ������ʵĽṹ���ʿ����жϣ�
��2������HCO3-Ҳ����NaOH����Һ��Ӧ��
��3���ٸ���ԭ�������ʱ�ʾĿ������������������������֮�����Ƚϣ�����ƽ�ⳣ��Խ��Խ�����ڷ�Ӧ�Ľ��У�
�ڷ�Ӧ���ȣ�����ֵ�����ݡ�G=��H-T?��S�жϣ�
�۸����¶ȶ�ƽ�ⳣ����Ӱ�죻
��4������ǡ�÷�Ӧ�����꣬����KMnO4��Һ��ɫ��ȥ�����ݻ�ѧ����ʽ4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O���HCHO�����ʵ�����Ȼ��������������������ȩ��Ũ�ȣ�
����⣺��1��a����ȩ�ж���ͬʱ����ʹ�����ʱ��ԣ�Ӱ�쵰���ʵ���������a����
b��װ�����к��д����ļ�ȩ�������к����ʣ��粻ͨ���ֱ����ס�����������ϴ�Σ������b��ȷ��
c��HCHO�е���̼��˫����NaHSO3�����ӳɷ�Ӧ����C��ȷ��
d����ȩ���ܶ���걾�еĵ����ʷ������ԣ��ƻ������ʵĽṹ�������걾�ĸ��ã���d��ȷ��
��ѡ��a
��2����HCO3-Ҳ����NaOH����Һ��Ӧ������NaOH����Һ��ʹ�òⶨ���ƫ�ߣ���ѡ��a��
��3������ԭ�������ʱ�ʾĿ������������������������֮�ȣ���ӦI��ԭ��������Ϊ
��100%=93.7%����ӦII��ԭ��������Ϊ
��100%=62.5%��ƽ�ⳣ��Խ��Խ�����ڷ�Ӧ�Ľ��У��ʴ�Ϊ��I��II��
����Ӧ���ȣ���H��0����ֵ����S��0�����ԡ�G=��H-T?��Sһ��С��0����Ӧ�Է�����ѡ��c��
�����¶����ߣ���ӦI�������ƶ���ƽ�ⳣ������ӦII�����ƶ���ƽ�ⳣ����С���ʴ�Ϊ��II��
��4��ǡ�÷�Ӧ�����꣬����KMnO4��Һ��ɫ��ȥ��
4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O
4 5
5��10-3 L��1.00��10-4mol/L n
n=6.25��10-7mol
��HCHO������Ϊ6.25��10-7mol��30g/mol=1.5��10-5g=1.875��10-2mg�����������Ϊ50L=0.05m3�����ڿ����м�ȩ��Ũ��
=0.375 mg/m3���ʴ�Ϊ������KMnO4��Һ��ɫͻȻ��ȥ��0.375��
b��װ�����к��д����ļ�ȩ�������к����ʣ��粻ͨ���ֱ����ס�����������ϴ�Σ������b��ȷ��
c��HCHO�е���̼��˫����NaHSO3�����ӳɷ�Ӧ����C��ȷ��
d����ȩ���ܶ���걾�еĵ����ʷ������ԣ��ƻ������ʵĽṹ�������걾�ĸ��ã���d��ȷ��
��ѡ��a
��2����HCO3-Ҳ����NaOH����Һ��Ӧ������NaOH����Һ��ʹ�òⶨ���ƫ�ߣ���ѡ��a��
��3������ԭ�������ʱ�ʾĿ������������������������֮�ȣ���ӦI��ԭ��������Ϊ
| 30 |
| 32 |
| 30 |
| 48 |
����Ӧ���ȣ���H��0����ֵ����S��0�����ԡ�G=��H-T?��Sһ��С��0����Ӧ�Է�����ѡ��c��
�����¶����ߣ���ӦI�������ƶ���ƽ�ⳣ������ӦII�����ƶ���ƽ�ⳣ����С���ʴ�Ϊ��II��
��4��ǡ�÷�Ӧ�����꣬����KMnO4��Һ��ɫ��ȥ��
4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O
4 5
5��10-3 L��1.00��10-4mol/L n
n=6.25��10-7mol
��HCHO������Ϊ6.25��10-7mol��30g/mol=1.5��10-5g=1.875��10-2mg�����������Ϊ50L=0.05m3�����ڿ����м�ȩ��Ũ��
| 1.875��10-2mg |
| 0.05m3 |
������������Ҫ�����˼�ȩ�����ʡ��Ʊ���ͬʱ������ʵ�飬�ѶȲ���֪ʶ��϶࣬��һ�����Ѷȣ�

��ϰ��ϵ�д�
 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
�����Ŀ
 ��2011?ɽ��ģ�⣩A��B��C��D��E��F�������ʵ��ת����ϵ��ͼ��ʾ����Ӧ����δ����������з�Ӧ�����û���Ӧ��
��2011?ɽ��ģ�⣩A��B��C��D��E��F�������ʵ��ת����ϵ��ͼ��ʾ����Ӧ����δ����������з�Ӧ�����û���Ӧ��
 ��2011?ɽ��ģ�⣩[��ѧ-ѡ���л���ѧ����]
��2011?ɽ��ģ�⣩[��ѧ-ѡ���л���ѧ����]


 ��2011?ɽ��ģ�⣩��ɣ��׳�������й�����������Ʒ��֮һ��ijͬѧ�������·��������Բⶨ��ͼ��ʾ�����ƿ���ݻ�����32.76g NaCl������뵽500ml�ձ��У�����200ml����ˮ����NaCl��ȫ�ܽ����Һȫ��ת�Ƶ���ƿ�У�������ˮϡ������ȫ��������������ȡ��100ml��Һ������Һǡ������10ml��0.100mol/L��AgNO3��Һ��ȫ��Ӧ��������˵����ȷ���ǣ�������
��2011?ɽ��ģ�⣩��ɣ��׳�������й�����������Ʒ��֮һ��ijͬѧ�������·��������Բⶨ��ͼ��ʾ�����ƿ���ݻ�����32.76g NaCl������뵽500ml�ձ��У�����200ml����ˮ����NaCl��ȫ�ܽ����Һȫ��ת�Ƶ���ƿ�У�������ˮϡ������ȫ��������������ȡ��100ml��Һ������Һǡ������10ml��0.100mol/L��AgNO3��Һ��ȫ��Ӧ��������˵����ȷ���ǣ�������