��Ŀ����
��NA��ʾ�����ӵ�������ֵ������˵����ȷ����
| A��5.6 g Fe������ϡHNO3������Ӧ��ת�Ƶĵ�����Ϊ0.3 NA |
| B����1 L 0.1 mol/L AlCl3��Һ�к���0.1 NA��Al3+ |
| C����ij�ܱ�������ʢ��0.1 mol N2��0.3 mol H2����һ�������³�ַ�Ӧ�ɵ�0.2 NA��NH3���� |
| D����״���£�11.2 L���Ȼ�̼�к��е�C��Cl���ĸ���Ϊ2 NA |
A
�������������A��Fe������ϡHNO3������Ӧ�����������۵������ӣ���ȷ��B�������ӻᷢ��ˮ�⣬�����1 L 0.1 mol/L AlCl3��Һ�к���С��0.1 NA��Al3+������D�����Ǹ����淴Ӧ�������ܽ��е��ף�����D����״���£����Ȼ�̼��Һ�壬����ʹ��22.4L/mol������
���㣺������ԭ��ˮ�⡢ƽ����й�֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
��ѧ���ѷ���һ����������ӣ��仯ѧʽΪH3������ͬ�����£���������H3��H2��ͬ���ǣ� ��
| A��ԭ���� | B�������� | C����� | D�����ʵ��� |
þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ��������������(V)��ʱ��(t)��ϵ��ͼ����Ӧ��þ������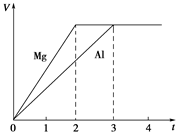
| A�����ʵ���֮��Ϊ3��2 |
| B������֮��Ϊ3��2 |
| C��Ħ������֮��Ϊ2��3 |
| D����Ӧ����֮��Ϊ2��3 |
����I����Cl����ϡ��Һ�е���AgNO3��Һ�����������������AgNO3��Һ����Ĺ�ϵ��ͼ��ʾ����ԭ��Һ��c(I��)/c(Cl��)�ı�ֵΪ( ) 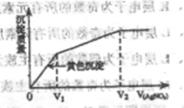
| A��(V2��V1)V1 | B��V1/V2 |
| C��V1/(V2��V1) | D��V2/V1 |
��NA��ʾ����٤����������ֵ������˵������ȷ���ǣ� ��
| A��1 L1mol/L H2SO4������2NA��H+ |
| B��500mL2mol/L�����100mL2mol/L����ĵ�����ǿ |
| C��1mol Na2S2����NA�����ۼ� |
| D����״���£�3��36 LSO2������ԼΪ9��6g |
��NA��ʾ�����ӵ�������ֵ�����������в���ȷ����
| A��16. 9 g���Ȼ���(BaO2)��������������������ԼΪ0. 2NA |
| B�����³�ѹ�£�28 g��ϩ�ͻ�����(C4H8)�Ļ�������к��е�̼ԭ����Ϊ2 NA |
| C��a gij���庬������Ϊb��c g�������ڱ���µ����Ϊ22. 4bc/ aNAL |
| D��ij�ܱ�����ʢ��0. l molN2��0. 3 mol H2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ0. 6NA |
��NA��ʾ�����ӵ�������ֵ������������ȷ����
| A��1mol Na2O2�������O22��2 NA |
| B�����³�ѹ�£�3.2 g CH4�к��е���2 NA |
| C����״���£�11.2 L CH3CH2OH�к��з���0.5NA |
| D��100 mL 1 mol��L-1��CH3COOH��Һ�к���CH3COOH����0.1 NA |
���л�ѧ������ȷ����
A�������ӵĽṹʾ��ͼ��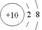 | B��CaH2�ĵ���ʽ��[H:]?Ca2+[:H]- |
C��CO2�ı���ģ�ͣ�  | D��������8�����ӵ�̼ԭ�ӣ�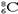 |
 2CaO+O2��+2nH2O��2CaO2+4HCl��2CaCl2+2H2O+O2�����ֳ�ȡ2.168 g����������Ʒ�ֳ����ȷݽ�������ʵ�飺
2CaO+O2��+2nH2O��2CaO2+4HCl��2CaCl2+2H2O+O2�����ֳ�ȡ2.168 g����������Ʒ�ֳ����ȷݽ�������ʵ�飺