��Ŀ����
20�������ķ��ӽṹ�У���ÿ����2����ԭ�ӣ����൱��̼̼������һ�Թ��õ��ӣ��Իش��������⣺��1������ʽΪCnH2n+2����������̼̼�乲�õ��Ӷ���Ϊn-1��
��2������ʽΪCnH2n-6����������̼̼�乲�õ��Ӷ���Ϊn+3��
��3��Cx�ɿ���Ϊ��ȥ���IJ����ij���ʷ�����̼̼�乲�õ��Ӷ���Ϊ160������ϸ�������̼���ʵĻ�ѧʽΪC80��
��4����ij��ϩ����̼̼�乲�õ��Ӷ���Ϊ80����õ�ϩ���ķ���ʽΪC80H160��
���� ÿ��̼ԭ����4���ۼ���������CxHy���ӣ�������ԭ��֮�����̼��̼֮��Ĺ��õ��Ӷԣ�����ÿ����̼������Щ���Ӷԣ����̼��̼֮��Ĺ��õ��Ӷ���Ϊ��$\frac{4x-y}{2}$�����ô˹�ʽ���뼴����⼴�ɣ�
��� �⣺ÿ��̼ԭ����4���ۼ���������CxHy���ӣ�������ԭ��֮�����̼��̼֮��Ĺ��õ��Ӷԣ�����ÿ����̼������Щ���Ӷԣ����̼��̼֮��Ĺ��õ��Ӷ���Ϊ��$\frac{4x-y}{2}$��
��1������ʽΪCnH2n+2����������̼̼�乲�õ��Ӷ���Ϊ��$\frac{4x-y}{2}$=$\frac{4n-��2n+2��}{2}$=n-1��
�ʴ�Ϊ��n-1��
��2������ʽΪCnH2n-6����������̼̼�乲�õ��Ӷ���Ϊ��$\frac{4x-y}{2}$=$\frac{4n-��2n-6��}{2}$=n+3��
�ʴ�Ϊ��n+3��
��3����ij���ʷ�����̼̼�乲�õ��Ӷ���Ϊ160����Cx�൱��CxHy������y=0����$\frac{4x}{2}$=160����ã�x=80��Cx�ķ���ʽΪC80��
�ʴ�Ϊ��C80��
��4����ϩ����ͨʽΪ��CnH2n����õ�ϩ��������̼̼�乲�õ��Ӷ���Ϊ��$\frac{4x-y}{2}$=$\frac{4n-2n}{2}$=80����ã�n=80���õ�ϩ���ķ���ʽΪ��C80H160��
�ʴ�Ϊ��C80H160��
���� ���⿼�����л������ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ��CxHy������̼��̼֮��Ĺ��õ��Ӷ���Ϊ$\frac{4x-y}{2}$Ϊ���ؼ�������������ѧ���ķ�����������������ѧ����������

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�| A�� | �������ˮ�Ļ������÷�Һ�ķ������� | |
| B�� | �������е���AgNO3��Һ�������е���Ԫ�أ�Br-+Ag+�TAgBr�� | |
| C�� | ȡ������ϡ���������µ�ˮ��Һ��һ�ྻ���Թ��У�����������Һ��ˮԡ���ȼ����������� | |
| D�� | ����Ȳʱ��������ʳ��ˮ��Ϊˮ�Եõ�ƽ�ȵ���Ȳ���� |
| A�� | �����ܶȴ���ˮ | |
| B�� | ���к���̼̼˫�������Ա�����ϩ�� | |
| C�� | ��������6��̼̼����ȫ��ͬ | |
| D�� | ����������ˮ�����������Һ��Ӧ��ʹ������ɫ |
| A�� | �ױ������ױ������ڱ���ͬϵ����������������ʷ����о����м� | |
| B�� | ����Ĺ������Ǹ��������б���̼ԭ��ֱ���������ǻ� | |
| C�� | ����ͷ��������ͬ�Ĺ����ţ����������ͬ�Ļ�ѧ���� | |
| D�� | �״����Ҷ��������Ͷ����ڴ������������������ʷ����о����б������� |
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | ����ʽΪC4H8��ϩ����4��ͬ���칹�壬���ǵ��۵㡢�е������ͬ | |
| B�� | ��ϩ�����ӡ���ȩ��������ˮ��Ӧ���䷴Ӧԭ��������ͬ | |
| C�� | ����߷��Ӳ���PPV��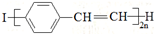 �;۱���ϩ������ͬ���ظ��ṹ��Ԫ �;۱���ϩ������ͬ���ظ��ṹ��Ԫ | |
| D�� | �Ե���Ϊ����ԭ�Ͽ���ȡ�������� |
| A�� | 28g��ϩ��30g�����к��еļ��Թ��ۼ�����֮��2��3 | |
| B�� | ���¡���ѹ�£�22.4L C2H4������ȫ�ӳ���ҪlmolBr2 | |
| C�� | C2H6��C4H10��һ����ͬϵ�� | |
| D�� | 1molH2O2��ȫ�ֽ�ʱת�Ƶ���2NA�� |
| A�� | 1.0mol/L ��Na2CO3��Һ�У�c��H+��+c��Na+��=c��HCO3-��+c��CO32-��+c��OH-�� | |
| B�� | 1.0mol/LNH4Cl��Һ��c��NH4+��=c��Cl-�� | |
| C�� | ���������Һ�м����������ᣬ�õ������Ի����Һ��c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| D�� | ����������Һ�еμ�ϡ����õ���pH=5�Ļ����Һ��c��Na+��=c��NO3-�� |