��Ŀ����
ij�����Һ�п��ܺ������������е������֣�NH4+��Na+��Al3+��Fe2+��Fe3+��AlO2-��SO42-��I-��NO3-��CO32-,��֪�����ӵ�Ũ�Ⱦ�ԼΪ0.1mol/L����ȡ����20mL����Һ��������ʵ�飺
��1����һ�ݼ����������ᣬ���������ڿ����б�Ϊ��ɫ���ڷ�Ӧ�����Һ�м���BaCl2��Һ���а�ɫ����������
��2���ڶ�����εμ�NaOH��Һ���������������������ܽ⣬���������ݳ���
��������ʵ�����������˵��������ǣ� ����
| A���û����Һ��һ��������Fe3+��AlO2-��Al3+��CO32- |
| B���û����Һͨ������������ɫ��Ӧ���Լ��ȷ���Ƿ���I- |
| C���û����Һ�п϶�����Fe2+��NH4+��SO42-��NO3- |
| D����û����Һ�еμӷ�̪��Һ��ʺ�ɫ |
D
���������������һ�ݼ����������ᣬ���������ڿ����б�Ϊ��ɫ��˵����Һ����NO3?�ͻ�ԭ������Fe2+��I?���ڷ�Ӧ�����Һ�м���BaCl2��Һ���а�ɫ����������˵������SO42?���ڶ�����εμ�NaOH��Һ���������������������ܽ⣬���������ݳ���˵������Al3+������NH4+��Fe2+��Fe3+�������ӵ�Ũ�Ⱦ�ԼΪ0.1mol/L����֪�û����Һ�п϶�����Fe2+��NH4+��SO42-��NO3-��һ��������Fe3+��AlO2-��Al3+��CO32-����A��C��ȷ��ͨ������������ɫ��Ӧ�ɼ����Ƿ���Na+��������Na+������I?��������Na+������I?����B����ȷ��D����ΪFe2+��I?ˮ�⣬��Һ�����ԣ��μӷ�̪��Һ��Ϊ��ɫ������
���㣺���⿼�����ӵ��ƶϡ�

 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д����и��������У���Ϊ��Ӧ������������ͬ��������ͬ��ѧ��Ӧ����( )
��C��O2 ��Na��O2 ��Fe��Cl2 ��AlCl3��Һ�백ˮ
��CO2��NaOH��Һ ��Cu������ ��AgNO3��Һ�백ˮ
| A�������� | B�����ۢ��� | C�����ۢ��� | D�����ޢ��� |
����������ʵ�ķ���ʽ��ȷ����
A�����ȿ���ǿ������Һȥ����: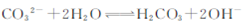 |
B����ϡ����ϴ������������Ӧ���Թ�: 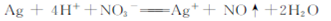 |
C����ú�м���ʯ��ʯ�ɼ���úȼ��ʱSO2���ŷ�: 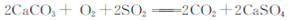 |
| D��̼��������Һ����������ʯ��ˮ��ϳ��ְ�ɫ����: |
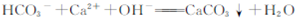
��25��������£����и�������һ������ָ�������д����������
A����c(H+)=10-10 mol/L����Һ�У� Al3+��NH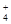 ��Cl�� ��NO ��Cl�� ��NO |
B��pH=13����Һ�У�K+ ��Na+��SO ��Cl�� ��Cl�� |
C��pH=2����Һ�У� K+��NH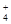 ��Cl����ClO�� ��Cl����ClO�� |
D�����ȳʺ�ɫ����Һ�У�Fe3+��Na+ ��SO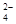 ��CO ��CO |
�����£����и��������ܴ����������
| A��ϡ�����У�K����Mg2+��AlO2����S2O32�� |
| B��Na2S��Һ�У�SO42-��K����Cl����Cu2�� |
C��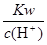 =10-13mol��L-1��Һ�У�Fe3+��NH4����Mg2+�� SO42- =10-13mol��L-1��Һ�У�Fe3+��NH4����Mg2+�� SO42- |
| D��ͨ�����CO2����Һ�У�Na+��ClO����CH3COO����HCO3�� |
����ָ����Ӧ�����ӷ���ʽ��ȷ����
| A��Cu����ϡ���Cu��2H����NO3��=Cu2����NO2����H2O |
| B��(NH4)2Fe(SO4)2��Һ�����NaOH��Һ��Ӧ��Fe(OH)2��Fe2����2OH��=Fe(OH)2�� |
| C����CH3COOH�ܽ�CaCO3��CaCO3��2H��=Ca2����H2O��CO2�� |
| D����NaAlO2��Һ��ͨ�����CO2��Al(OH)3��CO2��AlO2����2H2O=Al(OH)3����HCO3�� |
���и������µ��������һ���ܴ���������ǣ� ��
| A��c(HCO3��)=1��10��1mol/L����Һ��Na+��AlO2����CH3COO����K+ |
| B����ˮ�������c(H+)=1��10��14mol/L����Һ��CO32����NH4+��SO42����K+ |
| C�����ȳʺ�ɫ����Һ��Fe2+��Cl����NO3����Na+ |
| D����ʹpH��ֽ�ʺ�ɫ����Һ��Mg2+��Cl����NO3����SO42�� |
ij��Һ�д��ڴ�����H+��Cl����Fe3+������Һ�л����ܴ������ڵ�������
| A��OH�� | B��Ag+ | C��CO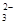 | D��SO |
