��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣��ޱ��е�λ�ã��û�ѧ����ش����⣺
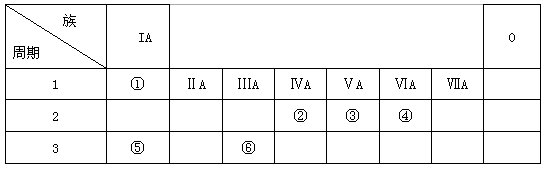
��1���ڢۢ����γ��⻯����ȶ��ԣ�д��ѧʽ��_____________
��2���� ���ʡ��ܵ��ʺ͢ݵ�����������Ӧˮ�����Ũ��Һ���ԭ��أ�д���������ϵĵ缫��Ӧ��������____________����_________________
��3����֪���ڡ��ĵ��ʸ�1 mol��ȫȼ�գ��ֱ�ų�����a kJ��b kJ�� �����ܺ͢ڵ������������û���Ӧ���Ҹ��û���ӦΪ���ȷ�Ӧ��д�����û���Ӧ���Ȼ�ѧ����ʽ___________________________________
��2���� ���ʡ��ܵ��ʺ͢ݵ�����������Ӧˮ�����Ũ��Һ���ԭ��أ�д���������ϵĵ缫��Ӧ��������____________����_________________
��3����֪���ڡ��ĵ��ʸ�1 mol��ȫȼ�գ��ֱ�ų�����a kJ��b kJ�� �����ܺ͢ڵ������������û���Ӧ���Ҹ��û���ӦΪ���ȷ�Ӧ��д�����û���Ӧ���Ȼ�ѧ����ʽ___________________________________
��1��H2O>NH3>CH4
��2��������2H2��4e-+ 4 OH-= 4 H2O��������O2 + 4e-+ 2H2O = 4OH-
��3��4Al(s)��3CO2(g) ==2Al2O3(s)��3C(s) ��H����(4b-3a)kJ/mol ��Al(s)��3/4CO2(g) ==
1/2Al2O3(s)��3/4C(s) ��H����(b-3/4a)kJ/mol
��2��������2H2��4e-+ 4 OH-= 4 H2O��������O2 + 4e-+ 2H2O = 4OH-
��3��4Al(s)��3CO2(g) ==2Al2O3(s)��3C(s) ��H����(4b-3a)kJ/mol ��Al(s)��3/4CO2(g) ==
1/2Al2O3(s)��3/4C(s) ��H����(b-3/4a)kJ/mol

��ϰ��ϵ�д�
�����Ŀ








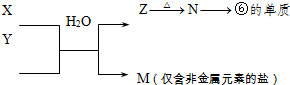
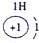
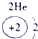
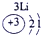
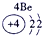
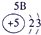
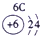
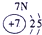
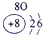
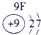
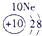
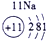





 ��ʾ����
��ʾ����