��Ŀ����
�±�ΪԪ�����ڱ���һ���֣�a��b��c��Ϊ����Ԫ�أ��ش��������⣺
��1����д������Ԫ��d3+�ĺ�������Ų�ʽ
��2����Ƚ�y��h��i����Ԫ�صĵ�һ�������ɴ�С��˳��
��3����д��eԪ�ص�ԭ�Ӽ۵����Ų�ͼ
 ��
��
��4��ya3���ӵĵ���ʽ��
 ������ӵĿռ乹����
������ӵĿռ乹����
��5��b��e����Ԫ���У������Խ�ǿ����
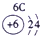
| a | |||||||||||||||||
| f | y | h | i | ||||||||||||||
| b | e | j | |||||||||||||||
| c | d | g | l | ||||||||||||||
1s222s2p63s23p63d5
1s222s2p63s23p63d5
����2����Ƚ�y��h��i����Ԫ�صĵ�һ�������ɴ�С��˳��
F��N��O
F��N��O
��дԪ�ط��ţ�����ԭ����ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ���NԪ��2p���Ӵﵽ��������ȶ����ͣ��������һ�����ܴ���OԪ��
ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ���NԪ��2p���Ӵﵽ��������ȶ����ͣ��������һ�����ܴ���OԪ��
����3����д��eԪ�ص�ԭ�Ӽ۵����Ų�ͼ


��4��ya3���ӵĵ���ʽ��


����
����
��������yԭ�ӵ�ԭ�ӹ�����ӻ�������sp3
sp3
����5��b��e����Ԫ���У������Խ�ǿ����
Mg
Mg
����Ԫ�ط��ţ���д����֤����һ���۵�һ��ʵ����ʵ������þ���Դ�����������
������þ���Դ�����������
������������Ԫ�����ڱ�֪��a��b��c��d��e��f��g��h��i��y��l�ֱ���H��Mg K Fe Al C Zn O F Br N Ga��
��1����Ԫ�������ڱ��е�λ�ÿ�֪��dΪFeԪ�أ�ԭ������Ϊ26��
��2��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��3��eΪ��Ԫ�أ����������Ӿ�����۵��ӣ�
��4��yΪN��aΪH����ya3����ΪNH3�����������ӵ���������
��5��bΪMg��eΪAl��Mg��Al���ڵ������ڣ�ͬ���ڴ������ҽ����Լ�����
��1����Ԫ�������ڱ��е�λ�ÿ�֪��dΪFeԪ�أ�ԭ������Ϊ26��
��2��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��3��eΪ��Ԫ�أ����������Ӿ�����۵��ӣ�
��4��yΪN��aΪH����ya3����ΪNH3�����������ӵ���������
��5��bΪMg��eΪAl��Mg��Al���ڵ������ڣ�ͬ���ڴ������ҽ����Լ�����
����⣺����Ԫ�����ڱ�֪��a��b��c��d��e��f��g��h��i��y��l�ֱ���H��Mg��K��Fe��Al��C��Zn��O��F��Br��N��Ga��
��1����Ԫ�������ڱ��е�λ�ÿ�֪��dΪFeԪ�أ�ԭ������Ϊ26����Fe3+�ĺ�������Ų�ʽ��1s222s2p63s23p63d5��
�ʴ�Ϊ��1s222s2p63s23p63d5��
��2��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ���NԪ��2p���Ӵﵽ��������ȶ����ͣ��������һ�����ܴ���OԪ�أ���������Ԫ�صĵ�һ�����ܴ�С˳����F��N��O��
�ʴ�Ϊ��F��N��O��
��3��eΪ��Ԫ�أ����������Ӿ�����۵��ӣ�������۵��ӹ����ʾʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4��yΪN��aΪH����ya3����ΪNH3�������ʽΪ ���ռ乹��Ϊ�����Σ��ӻ���ʽΪsp3��
���ռ乹��Ϊ�����Σ��ӻ���ʽΪsp3��
�ʴ�Ϊ�� �������Σ�sp3��
��������sp3��
��5��bΪMg��eΪAl��Mg��Al���ڵ������ڣ�ͬ���ڴ������ҽ����Լ���������þ�Ľ�����ǿ����֤���������ǿ���ķ���Ϊ��������þ���Դ�������������
�ʴ�Ϊ��Mg��������þ���Դ�������������
��1����Ԫ�������ڱ��е�λ�ÿ�֪��dΪFeԪ�أ�ԭ������Ϊ26����Fe3+�ĺ�������Ų�ʽ��1s222s2p63s23p63d5��
�ʴ�Ϊ��1s222s2p63s23p63d5��
��2��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ���NԪ��2p���Ӵﵽ��������ȶ����ͣ��������һ�����ܴ���OԪ�أ���������Ԫ�صĵ�һ�����ܴ�С˳����F��N��O��
�ʴ�Ϊ��F��N��O��
��3��eΪ��Ԫ�أ����������Ӿ�����۵��ӣ�������۵��ӹ����ʾʽΪ��
 ��
���ʴ�Ϊ��
 ��
����4��yΪN��aΪH����ya3����ΪNH3�������ʽΪ
 ���ռ乹��Ϊ�����Σ��ӻ���ʽΪsp3��
���ռ乹��Ϊ�����Σ��ӻ���ʽΪsp3���ʴ�Ϊ��
 ��������sp3��
�������Σ�sp3����5��bΪMg��eΪAl��Mg��Al���ڵ������ڣ�ͬ���ڴ������ҽ����Լ���������þ�Ľ�����ǿ����֤���������ǿ���ķ���Ϊ��������þ���Դ�������������
�ʴ�Ϊ��Mg��������þ���Դ�������������
���������⿼��Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã���ȷԪ�������ڱ��е�λ���ǽ����Ĺؼ���Ȼ���������ʽṹ������������ɣ��ѶȲ���

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ






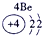
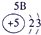
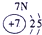
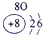
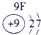
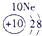
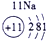





 ��ʾ����
��ʾ����