ƒøƒ⁄»ð
°æƒø°ø¢Ò.“—÷™¥◊À·∫Õ—ŒÀ· «»’≥£…˙ªÓ÷–º´Œ™≥£º˚µƒÀ·£¨‘⁄“ª∂®Ãıº˛œ¬£¨CH3COOH»Ð“∫÷–¥Ê‘⁄µÁ¿Î∆Ω∫‚:CH3COOH![]() CH3COO-+H+ °˜H>0°£
CH3COO-+H+ °˜H>0°£
(1)œ¬¡–∑Ω∑®÷–£¨ø…“‘ π0.10 mol/L CH3COOHµƒµÁ¿Î≥Ã∂»‘ˆ¥Ûµƒ «_______£®”√–Ú∫≈ÃÓ–¥£©°£
a.º”»Î…Ÿ¡ø0.10 mol/Lµƒœ°—ŒÀ· b.º”»»CH3COOH»Ð“∫
c.º”ÀÆœ° Õ÷¡0.010 mol/L d.º”»Î…Ÿ¡ø±˘¥◊À·
e.º”»Î…Ÿ¡ø¬»ªØƒ∆πÃàf.º”»Î…Ÿ¡ø0.10mol/LµƒNaOH»Ð“∫
(2)Ω´µ»÷ ¡øµƒ–ø¡£Õ∂»Îµ»Ãª˝«“pHæ˘µ»”⁄3µƒ¥◊À·∫Õ—ŒÀ·»Ð“∫÷–£¨æ≠π˝≥‰∑÷∑¥”¶∫Û£¨∑¢œ÷÷ª‘⁄“ª÷÷»Ð“∫÷–”––ø∑€ £”ý£¨‘Ú…˙≥…«‚∆¯µƒÃª˝πÿœµŒ™V£®—ŒÀ·£©_____V£®¥◊À·£©£®ÃÓ–¥°∞>°±°¢°∞<°±ªÚ°∞="£©.
(3)≥£Œ¬œ¬£¨œÚê˝Œ™VamL£¨pHŒ™3µƒ¥◊À·»Ð“∫÷–µŒº”pH=11µƒNaOH»Ð“∫VbmL÷¡»Ð“∫«°∫√≥ ÷––‘£¨‘ÚVa”ÎVbµƒπÿœµŒ™: Va______Vb£®ÃÓ–¥°∞>°±°¢°∞<°±ªÚ°∞=°±£©°£
(4)“—÷™: ƒ≥Œ¬∂» ±£¨ÀƵƒ¿Î◊”ª˝≥£ ˝Œ™Kw=1.0°¡10-12£¨‘⁄¥ÀŒ¬∂»œ¬£¨Ω´pH=1µƒ—ŒÀ·∫ÕpH=11µƒ«‚—ıªØƒ∆»Ð“∫µ»Ãª˝ªÏ∫œ£¨‘ÚªÏ∫œ»Ð“∫÷–µƒc(H+£©=_______mol/L°£
¢Ú.Ω·∫œœ¬±Ìªÿ¥œ¬¡–Œ £®æ˘Œ™≥£Œ¬œ¬µƒ ˝æ𣩣∫
À· | µÁ¿Î≥£ ˝£®Ka£© | À· | µÁ¿Î≥£ ˝£®Ka£© | À· | µÁ¿Î≥£ ˝£®Ka£© | À· | µÁ¿Î≥£ ˝£®Ka£© |
CH3COOH | 1.8°¡10-5 | H2CO3 | K1=4.4°¡10-7 | H2C2O4 | K1=5.4°¡10-2 | H2S | K1=1.3°¡10-7 |
HClO | 3°¡10-8 | K2=4.7°¡10-11 | K2=5.4°¡10-5 | K2=7.1°¡10-15 |
«Îªÿ¥œ¬¡–Œ Â:
(1) Õ¨≈®∂»µƒCH3COO-°¢HCO3/span>-°¢CO32-°¢HC2O4-°¢ClO-°¢S2-÷–Ω·∫œH+µƒƒÐ¡¶◊Ó»ıµƒ «_________°£
(2) 0.1mo1/LµƒH2C2O4»Ð“∫”Î0.1mo1/LµƒKOHµƒ»Ð“∫µ»Ãª˝ªÏ∫œ∫ÛÀ˘µ√»Ð“∫≥ À·–‘£¨∏√»Ð“∫÷–∏˜¿Î◊”≈®∂»”…¥ÛµΩ–°µƒÀ≥–ÚŒ™________________°£
(3)pHœýÕ¨µƒNaC1O∫ÕCH3COOK»Ð“∫÷–£¨[c(Na+)-c(C1O-)]______[c(K+)-c(CH3COO-)]£®ÃÓ°∞>°±°¢°∞<°±ªÚ°∞=°±£© °£
(4) œÚ0.1mo1/LCH3COOH »Ð“∫÷–µŒº”NaOH »Ð“∫÷¡c(CH3COOH): c(CH3COO-)=5:9£¨¥À ±»Ð“∫pH=_________°£
°æ¥∞∏°øbcf < < 1.0°¡10-6 HC2O4£≠ c(K£´)>c(HC2O4£≠)>c(H£´)>c(C2O42£≠)>c(OH£≠) £Ω 5
°æΩ‚Œˆ°ø
¢Ò(1)¥◊À·µƒµÁ¿Î «Œ¸»»∑¥”¶£¨º”ÀÆœ° Õ°¢º”ºÓ°¢º”»»∂ºƒÐ¥ŸΩ¯¥◊À·µƒµÁ¿Î£ª
(2)…˙≥…«‚∆¯µƒÃª˝»°æˆ”⁄µÁ¿Î≥ˆµƒ«‚¿Î◊”∂ý…Ÿ£ª
(3)ªÏ∫œ∫ۻГ∫«°∫√≥ ÷––‘£¨Àµ√˜«‚¿Î◊”∫Õ«‚—ı∏˘¿Î◊”µƒ≈®∂»œýµ»£ª
(4)«øÀ·∫Õ«øºÓªÏ∫œ∫ۻГ∫µƒ«‚¿Î◊”≈®∂»ø…∏˘æð÷–∫Õ∑¥”¶µƒ µ÷ ¿¥º∆À„£ª
¢Ú(1)À·∏˘¿Î◊”∂‘”¶µƒÀ·µƒµÁ¿Î∆Ω∫‚≥£ ˝‘Ω¥Û£¨Ω·∫œ«‚¿Î◊”ƒÐ¡¶‘Ω»ı£ª
(2)∑¥”¶∫Û»Ð÷ Œ™KHC2O4£¨À˘µ√»Ð“∫≥ À·–‘£¨Àµ√˜HC2O4-µƒµÁ¿Î≥Ã∂»¥Û”⁄∆‰ÀÆΩ‚≥Ã∂»£¨”÷«‚¿Î◊”¿¥◊‘ÀƵƒµÁ¿Î∫Õ≤ðÀ·«‚∏˘¿Î◊”µƒµÁ¿Î£¨‘Ú£∫c(H+)>c(C2O42-)>c(OH-)£ª
(3)∏˘æð¡Ω»Ð“∫÷–µƒµÁ∫… ÿ∫„∑÷Œˆ£ª
(4)∏˘æð¥◊À·µƒµÁ¿Î≥£ ˝±Ì¥Ô Ωº∞œýπÿ ˝æðΩ¯––º∆À„£¨«Û≥ˆ»Ð“∫÷–«‚¿Î◊”µƒ≈®∂»£ª
∏˘æð“‘…œ∑÷ŒˆΩ‚¥¥À°£
¢Ò(1) ¥◊À·µƒµÁ¿Î «Œ¸»»∑¥”¶£¨º”ÀÆœ° Õ°¢º”ºÓ°¢º”»»∂ºƒÐ¥ŸΩ¯¥◊À·µƒµÁ¿Î£ª
a. º”»Î…Ÿ¡ø0.10 mol/Lµƒœ°—ŒÀ·£¨»Ð“∫÷–«‚¿Î◊”≈®∂»‘ˆ¥Û£¨“÷÷∆¥◊À·µƒµÁ¿Î£¨‘Ú¥◊À·µƒµÁ¿Î≥Ã∂»ΩµµÕ£¨a¥ÌŒÛ£ª
b. ¥◊À·µƒµÁ¿Î «Œ¸»»∑¥”¶£¨º”»»CH3COOH»Ð“∫£¨Œ¬∂»…˝∏þ¥◊À·µƒµÁ¿Î∆Ω∫‚’˝œÚ“∆∂Ø£¨¥ŸΩ¯¥◊À·µƒµÁ¿Î£¨‘Ú¥◊À·µƒµÁ¿Î≥Ã∂»‘ˆ¥Û£¨b’˝»∑£ª
c.º”ÀÆœ° Õ£¨¥◊À·µƒµÁ¿Î∆Ω∫‚’˝œÚ“∆∂Ø£¨¥ŸΩ¯¥◊À·µƒµÁ¿Î£¨¥◊À·µƒµÁ¿Î≥Ã∂»‘ˆ¥Û£¨c’˝»∑£ª
d.º”»Î…Ÿ¡ø±˘¥◊À·£¨¥◊À·µƒµÁ¿Î∆Ω∫‚’˝œÚ“∆∂Ø£¨µ´¥◊À·µƒµÁ¿Î≥Ã∂»ΩµµÕ£¨d¥ÌŒÛ£ª
e.º”»Î…Ÿ¡ø¬»ªØƒ∆πÃࣨ≤ª”∞œÏ∆Ω∫‚µƒ“∆∂Ø£¨≤ª∏ƒ±‰¥◊À·µƒµÁ¿Î£¨e¥ÌŒÛ£ª
f.º”»Î…Ÿ¡ø0.10mol/LµƒNaOH»Ð“∫£¨OH-∫ÕH+∑¥”¶…˙≥…ÀÆ£¨«‚¿Î◊”≈®∂»ΩµµÕ£¨¥◊À·µƒµÁ¿Î∆Ω∫‚’˝œÚ“∆∂Ø£¨¥ŸΩ¯¥◊À·µƒµÁ¿Î£¨‘Ú¥◊À·µƒµÁ¿Î≥Ã∂»‘ˆ¥Û£¨f’˝»∑£ª
¥∞∏—°bcf°£
(2)¥◊À· «»ıÀ·£¨»Ð“∫÷–¥Ê‘⁄◊≈µÁ¿Î∆Ω∫‚£∫CH3COOH![]() CH3COO-+H+£¨º”»Î–ø∑€”ÎH+∑¢…˙∑¥”¶£¨∆Ω∫‚≤ª∂œœÚ”““∆∂Ø£¨ª·µÁ¿Î≥ˆ∏¸∂ýµƒH+ºÃ–¯”Ζø∑¥”¶…˙≥…«‚∆¯£¨“Ú¥Àµ»Ãª˝«“pHæ˘µ»”⁄3µƒ¥◊À·∫Õ—ŒÀ·»Ð“∫÷–£¨¥◊À·µƒ≈®∂»æÕ“™±»—ŒÀ·µƒ≈®∂»¥Û£¨ªπƒÐµÁ¿Î≥ˆ∏¸∂ýµƒH+ºÃ–¯”Ζø∑¥”¶…˙≥…«‚∆¯£¨À˘“‘…˙≥…«‚∆¯µƒÃª˝πÿœµŒ™V(—ŒÀ·£©< V(¥◊À·£©°£
CH3COO-+H+£¨º”»Î–ø∑€”ÎH+∑¢…˙∑¥”¶£¨∆Ω∫‚≤ª∂œœÚ”““∆∂Ø£¨ª·µÁ¿Î≥ˆ∏¸∂ýµƒH+ºÃ–¯”Ζø∑¥”¶…˙≥…«‚∆¯£¨“Ú¥Àµ»Ãª˝«“pHæ˘µ»”⁄3µƒ¥◊À·∫Õ—ŒÀ·»Ð“∫÷–£¨¥◊À·µƒ≈®∂»æÕ“™±»—ŒÀ·µƒ≈®∂»¥Û£¨ªπƒÐµÁ¿Î≥ˆ∏¸∂ýµƒH+ºÃ–¯”Ζø∑¥”¶…˙≥…«‚∆¯£¨À˘“‘…˙≥…«‚∆¯µƒÃª˝πÿœµŒ™V(—ŒÀ·£©< V(¥◊À·£©°£
¥∞∏Œ™£∫< °£
(3) ¥◊À· «»ıÀ·£¨pHŒ™3µƒ¥◊À·»Ð“∫÷–¥◊À·µƒ≈®∂»¥Û”⁄10-3mol/L£¨pH=11µƒNaOH»Ð“∫≈®∂»µ»”⁄10-3mol/L£¨ªÏ∫œ∫ۻГ∫«°∫√≥ ÷––‘£¨Àµ√˜ºÓ∂ý£¨º¥Va<Vb°£
¥∞∏Œ™£∫< °£
(4)pH=1µƒ—ŒÀ·÷–£¨c(H+)=0.1mol/L£¨pH=11µƒ«‚—ıªØƒ∆»Ð“∫÷–£¨c(OH-)=![]() =0.1mol/L£¨ªÏ∫œ∫Û∑¢…˙∑¥”¶H++ OH-=H2O£¨”…”⁄c(H+)= c(OH-)£¨‘ÚΩ´Ω´pH=1µƒ—ŒÀ·∫ÕpH=11µƒ«‚—ıªØƒ∆»Ð“∫µ»Ãª˝ªÏ∫œ£¨ªÏ∫œ“∫≥ ÷––‘£¨ªÏ∫œ»Ð“∫÷–µƒc(H+£©=
=0.1mol/L£¨ªÏ∫œ∫Û∑¢…˙∑¥”¶H++ OH-=H2O£¨”…”⁄c(H+)= c(OH-)£¨‘ÚΩ´Ω´pH=1µƒ—ŒÀ·∫ÕpH=11µƒ«‚—ıªØƒ∆»Ð“∫µ»Ãª˝ªÏ∫œ£¨ªÏ∫œ“∫≥ ÷––‘£¨ªÏ∫œ»Ð“∫÷–µƒc(H+£©=![]() mol/L=110-6mol/L°£
mol/L=110-6mol/L°£
¥∞∏Œ™£∫110-6°£
¢Ú(1)∆Ω∫‚≥£ ˝‘Ω¥Û£¨‘Ú∆‰Ω·∫œ«‚¿Î◊”ƒÐ¡¶‘Ω»ı£¨”…”⁄µÁ¿Î∆Ω∫‚≥£ ˝H2C2O4>HC2O4->CH3COOH>H2CO3>H2S>HClO>HCO3->HS-£¨‘ÚÕ¨≈®∂»µƒCH3COO-°¢HCO3-°¢CO32-°¢HC2O4-°¢ClO-°¢S2-÷–Ω·∫œH+µƒƒÐ¡¶”…»ıµΩ«øµƒÀ≥–ÚŒ™£∫HC2O4-°¢CH3COO-°¢HCO3-°¢ClO-°¢CO32-°¢S2-£¨Ω·∫œH+µƒƒÐ¡¶◊Ó»ıµƒ «HC2O4-°£
¥∞∏Œ™£∫HC2O4-°£
(2) 0.1mo1/LµƒH2C2O4»Ð“∫”Î0.1mo1/LµƒKOHµƒ»Ð“∫µ»Ãª˝ªÏ∫œ£¨∑¥”¶∫Û»Ð÷ Œ™KHC2O4£¨À˘µ√»Ð“∫≥ À·–‘£¨Àµ√˜HC2O4-µƒµÁ¿Î≥Ã∂»¥Û”⁄∆‰ÀÆΩ‚≥Ã∂»£¨‘ŸΩ·∫œ«‚¿Î◊”¿¥◊‘ÀƵƒµÁ¿Î∫Õ≤ðÀ·«‚∏˘¿Î◊”µƒµÁ¿Î£¨‘Úc(H£´)>c(C2O42£≠)>c(OH£≠)£¨»Ð“∫÷–¿Î◊”≈®∂»¥Û–°Œ™£∫c(K£´)>c(HC2O4£≠)>c(H£´)>c(C2O42£≠)>c(OH£≠) °£
¥∞∏Œ™£∫c(K£´)>c(HC2O4£≠)>c(H£´)>c(C2O42£≠)>c(OH£≠) °£
(3)∏˘æðµÁ∫… ÿ∫„NaCl∫ÕCH3COOK»Ð“∫÷–∑÷±”–£∫c(Na+) + c(H£´) =c(C1O-)+ c(OH£≠)°¢
c(K+) + c(H£´) =c(CH3COO-)+ c(OH£≠)°£”…Â÷™NaClO∫ÕCH3COOK»Ð“∫÷–pHœýÕ¨£¨‘Ú¡Ω»Ð“∫÷–c(H£´)°¢c(OH£≠)∑÷±œýµ»£¨º¥¡Ω»Ð“∫÷–c(OH£≠) - c(H£´)“≤œýµ»£¨À˘“‘[c(Na+)-c(C1O-)]= c(OH£≠) - c(H£´) =[c(K+)-c(CH3COO-)]°£
¥∞∏Œ™£∫= °£
(4)”…µÁ¿Î∆Ω∫‚:CH3COOH![]() CH3COO-+H+ø…÷™£∫Ka=
CH3COO-+H+ø…÷™£∫Ka=![]() =1.810-5£¨œ÷”–c(CH3COOH):c(CH3COO-)=5:9£¨‘ڻГ∫÷–c(H+)=Ka
=1.810-5£¨œ÷”–c(CH3COOH):c(CH3COO-)=5:9£¨‘ڻГ∫÷–c(H+)=Ka![]() =1.810-5
=1.810-5![]() =10-5mol/L£¨À˘“‘»Ð“∫µƒpH=5°£
=10-5mol/L£¨À˘“‘»Ð“∫µƒpH=5°£
¥∞∏Œ™£∫5°£

 ¿ºÕ∞ŸÕ®∆⁄ƒ©ΩæÌœµ¡–¥∞∏
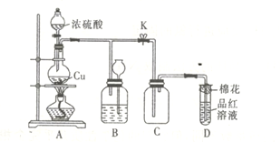
¿ºÕ∞ŸÕ®∆⁄ƒ©ΩæÌœµ¡–¥∞∏°æƒø°øƒ≥—–æø–‘—ßœ∞–°◊ÈŒ™¡ÀÃΩæø¥◊À·µƒµÁ¿Î«Èøˆ£¨Ω¯––¡À»Áœ¬ µ—È:
£®1£©»°±˘¥◊À·≈‰÷∆250 mL 0.4 mol°§L£≠1µƒ¥◊À·»Ð“∫£¨”√0.4 mol°§L£≠1µƒ¥◊À·»Ð“∫œ° Õ≥…À˘–Ë≈®∂»µƒ»Ð“∫£¨‘Ÿ”√NaOH±Í◊º»Ð“∫∂‘À˘≈‰¥◊À·»Ð“∫µƒ≈®∂»Ω¯––±Í∂®°£ªÿ¥œ¬¡–Œ £∫
¢ŸΩ´“ª∂®÷ ¡øµƒ±˘¥◊À·º”ÀÆœ° Õπ˝≥Ã÷–£¨»Ð“∫µƒµºµÁƒÐ¡¶±‰ªØ»Á”“ÕºÀ˘ æ°£‘Úœ° Õπ˝≥Ã÷–»Ð“∫µƒpH”…¥ÛµΩ–°µƒÀ≥–Ú______£®ÃÓ◊÷ƒ∏£©°£

¢⁄≈‰÷∆250 mL 0.4 mol°§L£≠1 ¥◊À·»Ð“∫ ±–Ë“™”√µΩµƒ≤£¡ß“«∆˜”–¡øÕ≤°¢…’±≠°¢≤£¡ß∞Ù°¢Ω∫Õ∑µŒπÐ∫Õ____________°£
¢€Œ™±Í∂®∏√¥◊À·»Ð“∫µƒ◊º»∑≈®∂»£¨”√0.2000 mol°§L£≠1µƒNaOH»Ð“∫∂‘20.00 mL¥◊À·»Ð“∫Ω¯––µŒ∂®£¨º∏¥ŒµŒ∂®œ˚∫ƒNaOH»Ð“∫µƒÃª˝»Áœ¬£∫
µ—È–Ú∫≈ | 1 | 2 | 3 | 4 |
œ˚∫ƒNaOH»Ð“∫µƒÃª˝(mL) | 20.05 | 20.00 | 18.80 | 19.95 |
∏√¥◊À·»Ð“∫µƒ◊º»∑≈®∂»Œ™_____________£®±£¡Ù–° ˝µ„∫ÛÀƒŒª£©£¨…œ ˆ±Í∂®π˝≥Ã÷–£¨‘Ï≥…≤‚∂®Ω·π˚∆´∏þµƒ‘≠“Úø…ƒÐ «_______________£®∂ý—°°¢¥Ì—°≤ªµ√∑÷£©°£
a£ÆŒ¥”√±Í◊º“∫»Ûœ¥ºÓ ΩµŒ∂®πÐ
b£ÆµŒ∂®÷’µ„∂¡ ˝ ±£¨∏© ”µŒ∂®πеƒøÃ∂»£¨∆‰À¸≤Ÿ◊˜æ˘’˝»∑
c£Æ ¢◊∞Œ¥÷™“∫µƒ◊∂–Œ∆ø”√’Ù¡ÛÀÆœ¥π˝£¨Œ¥”√¥˝≤‚“∫»Ûœ¥
d£ÆµŒ∂®µΩ÷’µ„∂¡ ˝ ±∑¢œ÷µŒ∂®πк‚◊Ï¥¶–¸π““ªµŒ»Ð“∫
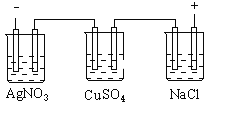
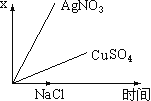
£®2£©∏√–°◊ÈÕ¨—ßÃΩæø≈®∂»∂‘¥◊À·µÁ¿Î≥Ã∂»µƒ”∞œÏ ±£¨”√pHº∆≤‚∂®25°Ê ±≤ªÕ¨≈®∂»µƒ¥◊À·µƒpH£¨∆‰Ω·π˚»Áœ¬£∫
¥◊À·≈®∂»( mol°§L£≠1) | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
¢Ÿ∏˘æð±Ì÷– ˝æð£¨ø…“‘µ√≥ˆ¥◊À· «»ıµÁΩ‚÷ µƒΩ·¬€£¨ƒ„»œŒ™µ√≥ˆ¥ÀΩ·¬€µƒ“¿æð «______________________________________________________________°£
¢⁄ºÚ ˆ”√pH ‘÷Ω≤‚ 0.1mol°§L£≠1 ¥◊À·»Ð“∫pHµƒ∑Ω∑®___________°£
¢€¿˚”√ÀÆΩ‚¿Ì¬€…˺∆ µ—È÷§√˜¥◊À·µƒÀ·–‘±»ÃºÀ·µƒ«ø£∫______________________°£
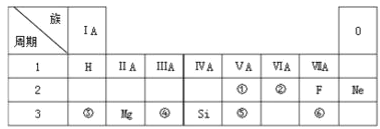
°æƒø°øœ¬±Ì «‘™Àÿ÷Ð∆⁄±Ìµƒ“ª≤ø∑÷£∫
¢ÒA | ¢ÚA | ¢ÛA | ¢ÙA | ¢ıA | ¢ˆA | ¢˜A | 0 | |
1 | A | |||||||
2 | D | E | G | I | ||||
3 | B | C | F | H |
(1)¥”‘≠◊”Ω·ππΩ«∂»∑÷Œˆ£¨D°¢E°¢G°¢IÀƒ÷÷‘™Àÿ¥¶”⁄Õ¨“ª––£¨ «”…”⁄À¸√«µƒ_______œýÕ¨°£E∫ÕF‘™Àÿ‘⁄÷Ð∆⁄±Ì¥¶”⁄Õ¨“ª¡– «”…”⁄À¸√«µƒ______œýÕ¨°£
(2)E‘™Àÿµƒ◊ÓµÕªØ∫œº€Œ™___________£¨‘≠◊”∞Îæ∂£∫r(E)_________ r(D)![]() °±°¢°∞
°±°¢°∞![]() °±ªÚ°∞
°±ªÚ°∞![]() °±
°±![]() £¨¿Î◊”∞Îæ∂£∫r(G)_________ r(C)(ÃÓ°∞
£¨¿Î◊”∞Îæ∂£∫r(G)_________ r(C)(ÃÓ°∞![]() °±°¢°∞
°±°¢°∞![]() °±ªÚ°∞
°±ªÚ°∞![]() °±)°£
°±)°£
(3)‘⁄±Ì÷–À˘¡–‘™Àÿ÷–◊Ó∏þº€—ıªØŒÔ∂‘”¶Àƪ،Ô÷–À·–‘◊Ó«øµƒ «________(–¥ªØ—ß Ω)°£
(4)ø∆—ߺ“Õ®π˝∂‘ƒ≥–©‘™ÀÿµƒªØ∫œŒÔΩ¯––—–æø£¨—∞’“∏þ–ß≈©“©°£’‚–©‘™ÀÿÕ˘Õ˘Œª”⁄‘™Àÿ÷Ð∆⁄±Ìµƒ _____________(—°ÃÓ–Ú∫≈)°£
a.◊Ûœ¬Ω««¯”Ú b.”“…œΩ««¯”Ú c.◊Û…œΩ««¯”Ú d.”“œ¬Ω««¯”Ú