��Ŀ����
[ ��ѧ����ѡ�����ʽṹ������][ѡ����]X��Y��Z��Q��E����Ԫ���У�Xԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�Yԭ�Ӻ����L���������K���������Z�ǵؿ��ں�����������������ߵ�Ԫ�أ�Q�ĺ˵������X��Z�ĺ˵����֮�ͣ�E��Ԫ�����ڱ��ĸ�Ԫ���е縺�������ش��������⣺
(1)X��Y��Ԫ�ط�������Ϊ �� ��
(2)XZ2��YZ2���ӵ�����ṹ�ֱ��� �� ����ͬ������������ˮ�е��ܽ�Ƚϴ���� ��д����ʽ���������� ��
(3)Q��Ԫ�ط����� �������ڵ� ���ڣ����ĺ�������Ų�ʽΪ �����γɻ�����ʱ��������ϼ�Ϊ ��
(4)�������ʾʽд��E���⻯����Һ�д��ڵ�������� ��
��1��S C
��2��V�� ֱ���� SO2 ��ΪCO2�ǷǼ��Է��ӣ�SO2��H2O���Ǽ��Է��ӣ����ݡ��������ܡ�ԭ����SO2��H2O�е��ܽ�Ƚϴ�
��3��Cr �� 1s22s22p63s23p63d54s1 +6
��4��F��H��F F��H��O O��H��F O��H��O
�������ɡ�Xԭ�Ӻ����M����ֻ�����ԳɶԵ��ӡ���XΪ��Ԫ�ء���Yԭ�Ӻ����L���������K�����������YΪ̼Ԫ�ء���Z�ǵؿ��ں�����ߵ�Ԫ�ء���ZΪ��Ԫ�ء���Q�ĺ˵������X��Z�ĺ˵����֮�͡���QΪ��Ԫ�أ���Ԫ�ط���ΪCr��λ��Ԫ�����ڱ��ĵ������ڣ����������Ų�ʽΪ��1s22s22p63s23p63d54s1�����γɻ�����ʱ������ϼ�Ϊ+6����E��Ԫ�����ڱ��ĸ�Ԫ���е縺�����EΪ����F��Ԫ�أ���E���⻯�HF����Һ�д��ڵ��������Ϊ��F��H��O��O��H��F��O��H��O��XZ2��YZ2�ֱ�ΪSO2��CO2���ӣ�������ṹ�ֱ�ΪV�Σ����Σ���ֱ���Σ�����ͬ�����¶�����ˮ���ܽ�Ƚϴ����SO2��ԭ����CO2�ǷǼ��Է��ӣ�SO2��H2O���Ǽ��Է��ӣ����ݡ��������ܡ�ԭ����SO2��H2O���ܽ�Ƚϴ�

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�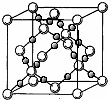 ����ѧ--ѡ�����ʽṹ�����ʡ�
����ѧ--ѡ�����ʽṹ�����ʡ�
 ��2011?֣�ݶ�ģ��[��ѧ--ѡ�����ʽṹ������]
��2011?֣�ݶ�ģ��[��ѧ--ѡ�����ʽṹ������]
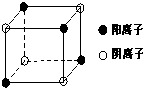 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�