��Ŀ����
����Ŀ�����л�ѧ�̲Ľ����˲��ֳ����Ľ�����ǽ���Ԫ�ؼ��仯��������֪ʶ���Իش���������
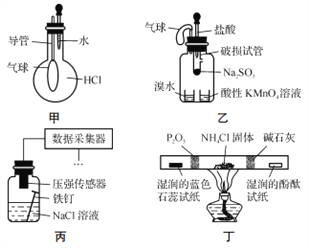
��1��ʵ������ȡ����������ʵ���У����˶������̡�Ũ�����Ũ���ᣬ����Ҫ___________��________����д�Լ�����Һ���ƣ�
��2��ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ��____________________
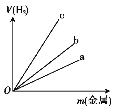
��3��ͼ���������߱�ʾп���������ֱ���ϡ���ᷴӦʱ����������H2����Ĺ�ϵ,�����ܱ�ʾ������ (�a����b����c��)��
��4����Ԫ�ص��⻯���H2O�⣬����H2O2��̼Ԫ�ص��⻯���CH4�⣬����C2H6�ȣ���Ԫ�ص��⻯���NH3�⣬���к�2����ԭ�ӵķ��ӵĻ�ѧʽΪ �����⻯�����������ᷴӦ�Ļ�ѧ��Ӧ����ʽΪ ��
���𰸡���ÿ��2������12����
��1�������Ȼ�����Һ��2�֣�������������Һ��2�֣�
��2��2ClO2��+Cl2=2ClO2+2Cl����2����
��3��c(2��)
��4��N2H4��2����N2H4+2HCl=N2H6Cl2��2����
��������
���������
��1��Ũ�����лӷ��ԣ���������ȡ�������к�������HCl���ñ���NaCl��Һ��ȥ����HCl�����������ж���Ϊ��ֹ��Ⱦ������Ӧ����NaOH��Һ���ա�����ʵ������ȡ���������������˶������̡�Ũ�����Ũ���ᣬ����Ҫ�����Ȼ�����Һ������������Һ��
��2������Cl2����NaClO2��Һ��ȡClO2������Cl2������������ԭ����ΪNaCl��ClO2Ϊ�����������˷�Ӧ�����ӷ�Ӧ����ʽΪ2ClO2-+Cl2=2ClO2+2Cl-��
��3����Al��Zn��Fe���ֽ����У�Al�Ľ�������ǿ������Al��ϡ���ᷴӦ��������죬����c���ߴ���Al��
��4��N���γ�3�����ۼ����������е���ʽ��һ��N�γɵ���NH3������N�γɵ���N2H4������ͬϵ����ʾ��������ԣ��ο�NH3�������N2H4��2HCl=N2H6Cl2��

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�����Ŀ��
����ͪ����ɫ��dz��ɫ��Һ�壬��ǿ�ҵĴ̼��Գ�ζ���ܶȣ����ˮ=1����0.95���۵㣺-45�����е㣺155�����ܽ�ȣ�100mL H2O����2.4g��31������
����Ӧ��
�ش��������⣺
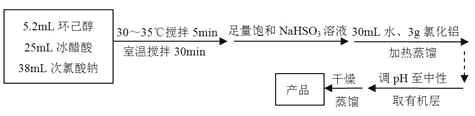
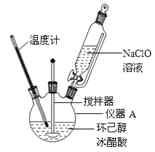
��1������A�������� ��
��2���ڷ�Ӧ��ʼ��5min��Ϊ�˽���ϵ�¶�ά����30��35���������ñ�ˮԡ��ȴ�⣬��ȡ�Ĵ�ʩ���� �� ��
��3�����뱥��NaHSO3��Һʱ��������Ҫ��Ӧ�� �������ӷ���ʽ��ʾ����ȷ������ı���NaHSO3��Һ�Ѿ�������ʵ������� ��
��4��Ϊ�����Һ��pH�������ԣ����Լ�����Լ��� ��
A��ϡ���� | B����ˮ̼���� | C��Ũ���� | D���������ƹ��� |
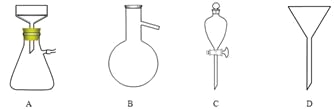
��5������pH������뾫��ʳ�Σ���Ŀ���� ����ȡ�л�����ʱʹ�õ���Ҫ������ ��������ͼ��Ӧ����ĸ����
��6��ʵ���������Ż���ʵ��ɰ�������Ҫ��С���Ա�����б�Ҫ��NaClO��Һ�����Ũ�Ƚ���̽��������������һϵ�в�ͬŨ�ȵ�NaClO��Һ�����õζ����궨�����巽���ǣ�����Һ��ȡ10.00mL NaClO��Һ��500mL����ƿ�ж��ݣ�ȡ25.00mL����ƿ�У���������ϡ�����KI���壨����������0.1000mol/L Na2S2O3����Һ�ζ����յ㣨�õ�����Һ��ָʾ����������ƽ��ʵ��ƽ������Na2S2O3����Һ18.00mL����֪I2+2Na2S2O3==2NaI+Na2S4O6 �����NaClO��Һ��Ũ���� ��