��Ŀ����
��9�֣���0.2000mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����
�� ������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�� �̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0����0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ������� ��ش�
��1�����ϲ����д�����ǣ����ţ� ���ô�������ᵼ�²ⶨ���(�ƫ����ƫС������Ӱ�족)__________________
��2��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ������²ⶨ���(�ƫ����ƫС������Ӱ�족)___________________
��3���жϵζ��յ�������ǣ���ƿ����Һ�� ɫ��Ϊ ɫ���Ұ���Ӳ���ɫ��
��4����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ mL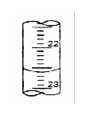
��5���±�������Ϊijͬѧ�����εζ�ʵ�������ã�������������������Ƿ�����������ǣ�����Ӧ�������ǵ� �Ρ�����д��ţ�
| �ζ����� | ���������ml�� | ���ռ������ml�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��1�� | 20.00 | 0.40 | 20.40 |
| ��2�� | 20.00 | 4.00 | 24.00 |
| ��3�� | 20.00 | 0.10 | 22.10 |
��1���٣�ƫ�� ��2��ƫС ��3���� ��dz�� ��4��22.60 ��5��3
���������������1������IJ����Ǣ٣��ζ���ϴ����֮��Ҫ��ϴ�������ʹ��Һ��Ũ��ƫС�����ʹ����Һ�����������ֵ�ⶨ�����ƫ�ߡ�
��2���ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ���ʹ��Һ������������ֵС�����Բⶨ���ƫС��
��3����������÷�̪��ָʾ�����ζ��յ�Ϊ��ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
��4����ͼ�п�֪�ζ��ܵĶ���Ϊ22.60mL��
��5���������Ͽ�֪�������ε�����ƫ��ϴ�
���㣺����к͵ζ�
����������dz���������Ҫ�������������͵ζ��еĻ���֪ʶ��

 ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�������������ˮϴ�Ӽ�ʽ�ζ��ܣ���ֱ��ע��NaOH��Һ����0���̶�������
ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�������������ˮϴ�Ӽ�ʽ�ζ��ܣ���ֱ��ע��NaOH��Һ����0���̶������� ��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺