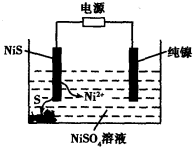
��Ŀ����
������������Ǻ���һ�����һ�������ˮƽ����Ҫ��־��
��1��һ�������£�SO2�������Ӧ10 min��SO2��SO3���ʵ���Ũ�ȷֱ�Ϊ1.2 mol/L��2.0 mol/L����SO2��ʼ���ʵ���Ũ��Ϊ______������SO3�Ļ�ѧ��Ӧ����Ϊ______��
��2�����ı��������������SO2�������Ӧ����SO3��ʹ10 min�ڵ���O2��ʾ�ķ�Ӧ����Ϊ0.15mol/(L��min)����ı������������_______________��
��3����ҵ�����ᣬ�ù����İ�ˮ��SO2β����������д����ص����ӷ���ʽ��____ _
��1��һ�������£�SO2�������Ӧ10 min��SO2��SO3���ʵ���Ũ�ȷֱ�Ϊ1.2 mol/L��2.0 mol/L����SO2��ʼ���ʵ���Ũ��Ϊ______������SO3�Ļ�ѧ��Ӧ����Ϊ______��
��2�����ı��������������SO2�������Ӧ����SO3��ʹ10 min�ڵ���O2��ʾ�ķ�Ӧ����Ϊ0.15mol/(L��min)����ı������������_______________��
| A��ѹ�����������ѹǿ | B�������¶� | C����������ĵ��� | D��������SO2��Ũ�� |
(1) 3.2 mol/L 0.2 mol/(L��min) ��(2) A D �� (3) SO2 + 2NH3��H2O = 2NH4��+ SO32��+ H2O
�����������1�����ݷ�Ӧ����ʽ2SO2(g) +O2(g)
 2SO3(g)��֪������SO3���ʵ���Ũ����2.0 mol/L�������ĵ�SO2�����ʵ���Ũ����2.0 mol/L����ʱ������SO2 1.2 mol/L�����Կ�ʼʱSO2�����ʵ���Ũ���ǣ�2.0 +1.2��mol/L="3.2mol/L." ����SO3�Ļ�ѧ��Ӧ����ΪV(SO3)=��c�¦�t="2.0" mol/L��10 min=" 0.2" mol/(L��min)����2��V(O2): V(SO3)=1:2������V(O2)= 0.1mol/(L��min).�������ı�����ʹV(O2)=" 0.15" mol/(L��min)��A��ѹ�����������ѹǿ����ʹ���ʵ�Ũ�������ܴﵽV(O2)= 0.1mol/(L��min)����ȷ��B���������¶ȣ���Ӧ���ʼ���������C����������ĵ����������������ݻ����䣬������ʵķ�Ӧ���ʲ��䡣����D��������SO2��Ũ�ȣ�����Ӧ���Ũ�Ȼ�ѧ��Ӧ���ʼӿ죬��ȷ����ѡ��ΪA��D����3��SO2������������ܹ�������Ӧ�����κ�ˮ������ڹ�ҵ�����ᣬ�����ù����İ�ˮ��SO2β�����������õ����Σ������Ӧ�����ӷ���ʽ��SO2 + 2NH3��H2O = 2NH4��+ SO32��+ H2O��
2SO3(g)��֪������SO3���ʵ���Ũ����2.0 mol/L�������ĵ�SO2�����ʵ���Ũ����2.0 mol/L����ʱ������SO2 1.2 mol/L�����Կ�ʼʱSO2�����ʵ���Ũ���ǣ�2.0 +1.2��mol/L="3.2mol/L." ����SO3�Ļ�ѧ��Ӧ����ΪV(SO3)=��c�¦�t="2.0" mol/L��10 min=" 0.2" mol/(L��min)����2��V(O2): V(SO3)=1:2������V(O2)= 0.1mol/(L��min).�������ı�����ʹV(O2)=" 0.15" mol/(L��min)��A��ѹ�����������ѹǿ����ʹ���ʵ�Ũ�������ܴﵽV(O2)= 0.1mol/(L��min)����ȷ��B���������¶ȣ���Ӧ���ʼ���������C����������ĵ����������������ݻ����䣬������ʵķ�Ӧ���ʲ��䡣����D��������SO2��Ũ�ȣ�����Ӧ���Ũ�Ȼ�ѧ��Ӧ���ʼӿ죬��ȷ����ѡ��ΪA��D����3��SO2������������ܹ�������Ӧ�����κ�ˮ������ڹ�ҵ�����ᣬ�����ù����İ�ˮ��SO2β�����������õ����Σ������Ӧ�����ӷ���ʽ��SO2 + 2NH3��H2O = 2NH4��+ SO32��+ H2O��
��ϰ��ϵ�д�
�����Ŀ
 2SO3 ���ƽ��ʱ����������ʵ���Ϊ5mol����
2SO3 ���ƽ��ʱ����������ʵ���Ϊ5mol����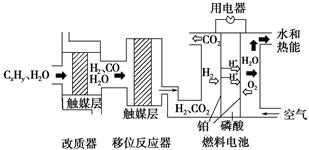
 CO2(g)��H2(g)����H��0�������¶�Խ�ߣ���v(CO)Խ��
CO2(g)��H2(g)����H��0�������¶�Խ�ߣ���v(CO)Խ�� CaO(s) + SO2(g) + CO2(g) ��H1=218.4kJ��mol-1(��Ӧ��)
CaO(s) + SO2(g) + CO2(g) ��H1=218.4kJ��mol-1(��Ӧ��) 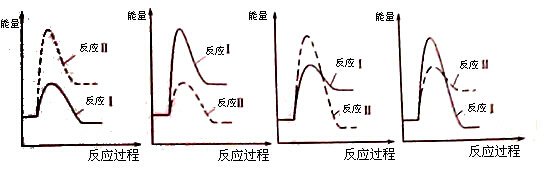
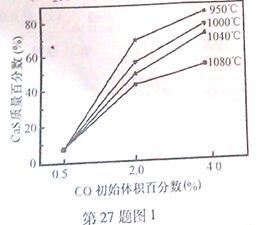
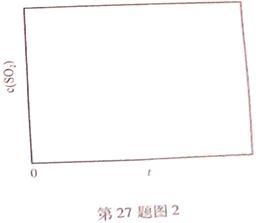

 ��
�� CH3OH+H2O����ش��������⣺
CH3OH+H2O����ش��������⣺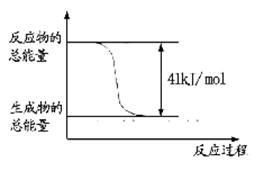
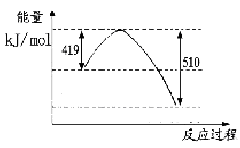 д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _��
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _��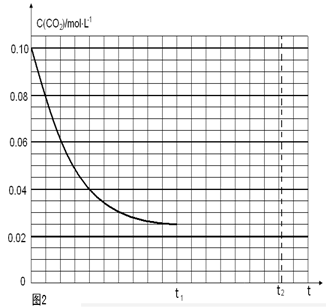

 ��_________���ƶ���
��_________���ƶ���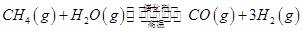 ��һ���¶��£���2 L�ݻ�������ܱ������г���4 mol
��һ���¶��£���2 L�ݻ�������ܱ������г���4 mol 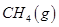 ��6 mo1 H2O(g)������Ӧ��10 minʱ����Ӧ�ﵽƽ��״̬�����CH4(g)��H2(g)�����ʵ�����ʱ��仯��������ͼ��ʾ��
��6 mo1 H2O(g)������Ӧ��10 minʱ����Ӧ�ﵽƽ��״̬�����CH4(g)��H2(g)�����ʵ�����ʱ��仯��������ͼ��ʾ��
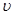 (CO)��ʾ�Ļ�ѧ��Ӧ����Ϊ_________��
(CO)��ʾ�Ļ�ѧ��Ӧ����Ϊ_________��
 Ni��CO��4��g��
Ni��CO��4��g��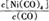 ������?H 0���>����<������
������?H 0���>����<������
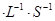 ��
�� )
)