��Ŀ����
����Ŀ��ij��ɫ��Һ������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �е���������ɡ�Ϊ��ȷ������Һ�ijɷ�, ��������ʵ�飺
�е���������ɡ�Ϊ��ȷ������Һ�ijɷ�, ��������ʵ�飺
�� ȡ��������Һ�������ϡ����,����������,�õ�����Һ;
�� ȡ��������Һ,�����м��������ữ����������Һ,���ְ�ɫ������;
�� ȡ����ԭ��Һ,�����м���Ba(OH)2��Һ,���ְ�ɫ�����ҡ�
��������ʵ��ش���������:
��1����Һ��һ�������ڵ�������___________________��
��2���������ڵ�������______________________��
��3����������һ������_______________________ ,���ܺ���________________________��
�ж��ҳɷֵķ�����______________________________________________��
�йط�Ӧ�����ӷ���ʽ____________________________________________��
���𰸡� MnO4-��Cu2+��Ag+��Ba2+��Al3+ Na+��CO32- BaCO3 BaSO4 �ڳ������м�������ϡ���ᣬ������ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ���� BaCO3+2H+= Ba2+ + H2O+CO2��
����������ɫ��Һ���ų� Cu2����MnO4��
1���������ϡ���ᣬ���������ɣ�����һ������CO32�D ����Ag����Ba2����Al3������Ϊ��Щ������CO32����Ӧ�õ�������
2��ȡ��������Һ�������м��������ữ����������Һ�����ְ�ɫ���������п�������Ϊ��һ���м����Cl�����µģ�������ȷ��ԭ��Һ�Ƿ���Cl��
3������������ȷ����Һ�к���CO32�D ������Һ�б���Ҫ����������ֻ����Na����.����һ����Na����
���ۣ���1����Һ��һ�������ڵ�������MnO4-��Cu2+��Ag+��Ba2+��Al3+����2��һ�����е�����CO32����Na������һ�����е����� Cl������3����������һ������ BaCO3�����ܺ���BaSO4���ж��ҳɷֵķ������ڳ������м�������ϡ���ᣬ������ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ����йط�Ӧ�����ӷ���ʽBaCO3+2H+= Ba2+ + H2O+CO2����

����Ŀ��X��Y��Z��W��U����Ԫ�أ���λ�����ڱ���ǰ�����ڣ����ǵĺ˵�����������ӣ��Һ˵����֮��Ϊ54��X�ļ���̬ԭ�ӵ����Ų�ʽΪ2p1,Yԭ�ӵĻ�̬ԭ����3����ͬ���ܼ����Ҹ��ܼ��е�������ȣ�Z�Ļ�̬ԭ������3��δ�ɶԵ��ӣ�W�ĵ��������±���ʾ��Uԭ�ӵ�K�������������������֮��Ϊ2:1����d�������ȫ����״̬��
Ԫ�� | ��һ������ | �ڶ������� | ���������� |
W | 495.8 | 4562 | 6910.3 |
��1��Uԭ�ӵ����Ų�ʽΪ_______________��
��2��X��Z �γɵĻ�����Z2X4��Zԭ�Ӳ�ȡ���ӻ��������Ϊ_______,Y������������ˮ��������ε������ӵĿռ乹��Ϊ_______��
��3��W���Ȼ����Mg���Ȼ����У��۵�ϸߵ���________���ѧʽ����ԭ����_______��
��4��I4O9��һ�������Ӻ�һ�������Ӱ����ʵ�����1:3���ɣ������ӵĿռ乹��Ϊ�����Σ�����ԭ���ӻ�����Ϊsp3�ӻ���д��I4O9�ĵ��뷽��ʽ______��
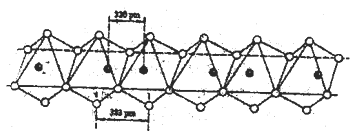
��5��Nb������I���������γ�һ�����Ե����߷��ӻ������ṹ�����������ͨ����߶����������ij���������ͼ��ʾ����û�������Nb��I��ԭ�Ӹ�����Ϊ______��