ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ»γΆΦ «≤ΩΖ÷ΕΧ÷ήΤΎ‘ΣΥΊΒΡΒΞ÷ ΦΑΤδΜ·ΚœΈοΒΡΉΣΜ·ΙΊœΒΆΦΘ®”–ΙΊΖ¥”ΠΒΡΧθΦΰΦΑ…ζ≥…ΒΡH2O“―¬‘»ΞΘ©
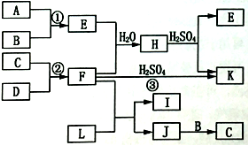
ΦΚ÷ΣΘΚ
Θ®aΘ©AΓΔBΓΔCΓΔD «Ζ«Ϋπ τΒΞ÷ Θ§Τδ÷–BΓΔCΓΔD‘Ύ≥ΘΈ¬≥Θ―Ιœ¬ «ΤχΧεΓΘ
Θ®bΘ©Ζ¥”ΠΔΌΓΔΔΎ «Μ·ΙΛ…ζ≤ζ÷–ΒΡ÷Ί“ΣΖ¥”ΠΓΘ
Θ®cΘ©Μ·ΚœΈοE «–Έ≥…Υα”ξΒΡΈέ»ΨΈο÷°“ΜΘ§Μ·ΚœΈοK «≥Θ”ΟΒΡΒΣΖ ΓΘ
Θ®dΘ©Μ·ΚœΈοLΨΏ”–Τ·ΑΉ–‘Θ§Ω…”…Cl2”κNaOH»ή“ΚΖ¥”ΠΕχ÷ΤΒΟΓΘ
Θ®eΘ©Μ·ΚœΈοJ”…ΝΫ÷÷‘ΣΥΊΉι≥…Θ§ΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ32ΓΘ
«κΑ¥“Σ«σΧνΩ’ΘΚ
Θ®1Θ©Ζ¥”ΠΔέΒΡΜ·―ßΖΫ≥Χ Ϋ____________________ΓΘ
Θ®2Θ©CΒΡΫαΙΙ Ϋ________ΘΜHΒΡΜ·―ß Ϋ_________ΓΘ
Θ®3Θ©LΒΡ»ή“Κ”κΜ·ΚœΈοEΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚΘ®Φν–‘ΧθΦΰΘ©_______________ΓΘ
Θ®4Θ©Μ·ΚœΈοJΒΡΜ·―ß Ϋ_______________ΓΘ
ΓΨ¥πΑΗΓΩΘ®1Θ©2NH3+H2SO4=(NH4)2SO4ΘΜ
Θ®2Θ©(NH4)2SO3ΘΜ
Θ®3Θ©ClO-+SO2+2OH-=Cl-+SO42-+H2OΘΜ
Θ®4Θ©N2H4ΓΘ
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚ”…ΕΧ÷ήΤΎ‘ΣΥΊΒΡΒΞ÷ ΦΑΤδΜ·ΚœΈοΒΡΉΣΜ·ΙΊœΒΆΦΩ…÷ΣΘ§Μ·ΚœΈοE «–Έ≥…Υα”ξΒΡΈέ»ΨΈο÷°“ΜΘ§‘ρEΈΣSO2Θ§AΓΔBΓΔCΓΔDΕΦ «Ζ«Ϋπ τΒΞ÷ Θ§Τδ÷–BΓΔCΓΔD‘Ύ≥ΘΈ¬≥Θ―Ιœ¬ «ΤχΧεΘ§‘ρAΈΣSΘ§BΈΣO2ΘΜΜ·ΚœΈοK «≥Θ”ΟΒΡΒΣΖ Θ§KΈΣΝρΥαοßΘ§Υυ“‘FΈΣΑ±ΤχΘ§‘ρCΓΔDΖ÷±πΈΣN2ΓΔH2ΘΜΜ·ΚœΈοLΨΏ”–Τ·ΑΉ–‘Θ§Ω…”…Cl2”κNaOH»ή“ΚΖ¥”ΠΕχ÷ΤΒΟΘ§‘ρLΈΣNaClOΘ§Α±Τχ”κNaClOΖ¥”Π…ζ≥…JΘ§J”…ΝΫ÷÷‘ΣΥΊΉι≥…Θ§Υϋ «ΜπΦΐΆΤΫχΦΝΒΡ≥…Ζ÷÷°“ΜΘ§ΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ32Θ§‘ρJΈΣN2H4ΘΜJ”κ―θΤχΖ¥”Π…ζ≥…CΘ§CΈΣΒΣΤχΓΘ
Θ®1Θ©ΗΟΖ¥”ΠΈΣΑ±Τχ”κΝρΥαΖ¥”Π…ζ≥…ΝρΥαοßΘ§Μ·―ßΖ¥”ΠΈΣ2NH3+H2SO4®TΘ®NH4Θ©2SO4Θ§Ι ¥πΑΗΈΣΘΚ2NH3+H2SO4®TΘ®NH4Θ©2SO4ΘΜ
Θ®2Θ©CΈΣΒΣΤχΘ§ΤδΫαΙΙ ΫΈΣNΓ‘NΘ§HΈΣΕΰ―θΜ·Νρ”κΑ±Τχ‘ΎΥ°÷–ΒΡΖ¥”ΠΘ§Ζ¥”Π…ζ≥…Θ®NH4Θ©2SO3ΜρNH4HSO3Θ§Ι ¥πΑΗΈΣΘΚNΓ‘NΘΜΘ®NH4Θ©2SO3ΜρNH4HSO3ΘΜ
Θ®3Θ©LΈΣNaClOΘ§EΈΣSO2Θ§‘Ύ»ή“Κ÷–ΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”Π…ζ≥…¬»Μ·ΡΤΓΔΝρΥαΡΤΘ§άκΉ”Ζ¥”ΠΈΣClO- + SO2 + 2OH- ®T Cl- + SO42- + H2OΜρClO- + SO2 + H2O ®T Cl- + SO42- + 2H+Θ§Ι ¥πΑΗΈΣΘΚClO-+SO2+2OH-®TCl-+SO42-+H2OΜρClO-+SO2+H2O®TCl-+SO42-+2H+ΘΜ
Θ®4Θ©”……œ ωΆΤΕœΩ…÷ΣΘ§JΈΣN2H4Θ§Ι ¥πΑΗΈΣΘΚN2H4ΓΘ




