��Ŀ����
����Ŀ�����õ�ζ����������һ�ִӻ���������ȡ��ˮ�����ӷ����㾫�ͣ������Ҵ������ѵ��л��ܼ���������ͼ��ʾװ�ô��������ѷۣ��������ᴿ�õ������͡�

ʵ�鲽�裺
��һ����Aװ���е�Բ����ƿ��װ��![]() �ݻ���ˮ����1~2����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
�ݻ���ˮ����1~2����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
����������Aװ���е�Բ����ƿ�����д�����������ʱ�رյ��ɼУ���������
�����������Һ�м���ʳ�������ͣ�����15mL������ȡ2�Σ���������ȡ���Ѳ�ϲ�������������ˮNa2SO4����Һ���㵹��������ƿ�У�����û����͡�
(1)װ��A�в����ܵ�������_______��װ��B��Բ����ƿ��б��Ŀ���� ________��
(2)���裨�����У����۲쵽_______����ʱ����ֹͣ�����������ʱ�����в�����˳��Ϊ_______�����ţ���
��ֹͣ���Ȣڴ��ɼТ۹ر�����ˮ
(3)�����Һ�м���ʳ�ε�������__ ��������ˮNa2SO4��������_______��
(4)ʵ���������ϡNaOH��Һ��ϴ�����ܣ���Ӧ�Ļ�ѧ����ʽΪ_________������������![]() ��ʾ��
��ʾ��
(5)Ϊ�ⶨ����������֬�ĺ�����ȡ20.00mL�����������Ҵ��У���80.00mL0.5mol/LNaOH���Ҵ���Һ�����裬��ַ�Ӧ����ˮ���200mL��Һ��ȡ25.00mL�����̪����0.1moI/L������еζ����ζ��յ���������20.00mL����û������к�����֬_______ g/L��
���� �ƣ�ʽ����884)��
�ƣ�ʽ����884)��
���𰸡�ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ���� ��ֹ�ɽ����Һ�������������(��������) �����״����Һ����״Һ�� �ڢ٢� �����ͻ�������ˮ�е��ܽ�ȣ������ڷֲ� ��ȥ�������е�ˮ����� 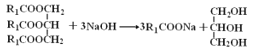 353.6g/L
353.6g/L
��������
��Aװ���м��Ȳ���ˮ������ˮ�����������ܽ���Bװ�ã���װ��B�л����ѷ���ˮ�Ļ������м�����ȡ�����ͣ������Һ�м���ʳ�ο������ɽ��ͻ�������ˮ�е��ܽ�ȣ����ڻ����ͷֲ����������ڻ����������ܽ����л��ܼ������У���������ˮ�������ܣ���������ȡ���к��еĻ����ͣ����������Ƴ�ȥ�Ѳ���������ˮ���������õ������͡����ݻ����͵���Ҫ�ɷ�������֬���ܹ���NaOH��Ӧ������֬�����ƺ��ͣ�������NaOH��HCl�ζ�����������к͵ζ���������к��еĻ����͵������������ɵû���������֬�ĺ�����
(1)����ʱ��ƿ������ѹǿ�������ܿɻ�������ѹǿ��ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ����װ��B��Բ����ƿ��б���Է�ֹ�ɽ����Һ�������������(��������)��
(2)����Aװ���е�Բ����ƿ�����д�����������ʱ�رյ��ɼУ���������װ��B�еĻ����ͻ������ȵ�ˮ�������ϱ�Ϊ�����������������״����Һ����״Һ�壬˵����������ȫ�����������ʱֹͣ�����������ʱ�������Ǵ��ɼУ�Ȼ��ֹͣ���ȣ����ر�����ˮ���ʲ�����˳��Ϊ�ڢ٢ۣ�
(3)�����Һ�м���ʳ�ε�����������ˮ����ܶȣ����ͻ�������ˮ�е��ܽ�ȣ������ڷֲ㣻������ˮNa2SO4����������ˮNa2SO4��ˮ����γ�Na2SO410H2O���Ա��ڳ�ȥ�������е�ˮ��Ի����ͽ��и��
(4)ʵ���������ϡNaOH��Һ��ϴ�������ڱ���մ�е���֬�����߷�����Ӧ���������Եĸ�֬�����ƺ��ͣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��
(5)����HCl+NaOH=NaCl+H2O������n(NaOH)(����)=n(HCl)=0.1mol/L��0.020L��![]() =0.016mol��������֬��Ӧ�����ʵ��������ʵ���Ϊ��0.5mol/L��0.08L-0.016mol=0.024mol�����ݻ�������NaOH��Ӧ�����ʵ��������ʵ�����ϵ��֪���к��еĻ����͵����ʵ���Ϊn(��֬)=
=0.016mol��������֬��Ӧ�����ʵ��������ʵ���Ϊ��0.5mol/L��0.08L-0.016mol=0.024mol�����ݻ�������NaOH��Ӧ�����ʵ��������ʵ�����ϵ��֪���к��еĻ����͵����ʵ���Ϊn(��֬)=![]() n(NaOH)=
n(NaOH)=![]() ��0.024mol=0.008mol��������Ϊm(��֬)=0.008mol��884g/mol=7.072g����û������к�����֬7.072g��0.02L=353.6g/L��
��0.024mol=0.008mol��������Ϊm(��֬)=0.008mol��884g/mol=7.072g����û������к�����֬7.072g��0.02L=353.6g/L��

 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�����Ŀ���ʰ�������![]() ��һ�ֲ���ǿ�����㷺����ȱ����ƶѪ��Ԥ�������ơ�ijѧϰС������������������Һ��ʰ��ᷴӦ�Ʊ��ʰ����������й��������������
��һ�ֲ���ǿ�����㷺����ȱ����ƶѪ��Ԥ�������ơ�ijѧϰС������������������Һ��ʰ��ᷴӦ�Ʊ��ʰ����������й��������������
�ʰ���( | ������ | �ʰ������� |
������ˮ�������Ҵ������Ի����� | ������ˮ���Ҵ�����ǿ���Ժͻ�ԭ�� | ������ˮ���������Ҵ� |
ʵ�����
ʵ��1�Ʊ�![]() ������
������![]() ����Һ��
����Һ��![]() ��Һ��ϣ���Ӧ��������˲�ϴ�ӳ�����
��Һ��ϣ���Ӧ��������˲�ϴ�ӳ�����
ʵ��2�Ʊ�![]() ��
��
ʵ��װ����ͼ(�гֺͼ���������ʡ��)����ʵ��1�õ��ij����ͺ�![]() �ʰ����ˮ��Һ��Ϻ����
�ʰ����ˮ��Һ��Ϻ����![]() �С�����
�С�����![]() �еķ�Ӧ��
�еķ�Ӧ��![]() �п����ž������ŵ�����������Һ�����ȡ�
�п����ž������ŵ�����������Һ�����ȡ�
ʵ��3�ᴿ![]() ����Ӧ�������������Һ����Ũ���������Ҵ������ˡ�����õ���Ʒ��
����Ӧ�������������Һ����Ũ���������Ҵ������ˡ�����õ���Ʒ��
(1)д��ʵ��1���Ʊ�![]() �����ӷ���ʽ_________________��
�����ӷ���ʽ_________________��
(2)װ���������������__________��װ��![]() �е��Լ���__________��
�е��Լ���__________��
(3)ʵ��2�У���![]() �ر�
�ر�![]() ���ž�������ȷ��
���ž�������ȷ��![]() �п����ž���ʵ��������________________���ž���������е�ʵ�������________�����ŵ�����������Һ�����ȡ�
�п����ž���ʵ��������________________���ž���������е�ʵ�������________�����ŵ�����������Һ�����ȡ�
(4)ʵ��2�е�����ɵ�����Һ![]() ����ϵ
����ϵ![]() ����ʵĹ�ϵ���
����ʵĹ�ϵ���
ʵ�� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
��ϵ | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 |
����(%) | 65.74 | 74.96 | 78.78 | 83.13 | 85.57 | 72.98 | 62.31 |
![]() ���Ͳ����½���ԭ����________________________������������û���_______��
���Ͳ����½���ԭ����________________________������������û���_______��
(5)ʵ��3�м����Ҵ���Ŀ����____________________��
(6)����Ʒ������Ϊ![]() �������Ϊ__________________%��
�������Ϊ__________________%��
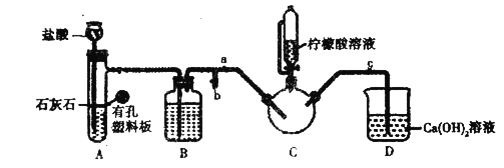
����Ŀ��CCl3CHO��һ��ҩ��ϳɵ��м��壬��ͨ��CH3CH2OH+4Cl2��CCl3CHO+5HCl�����Ʊ����Ʊ�ʱ���ܷ����ĸ���ӦΪC2H5OH+HCl��C2H5Cl+H2O��CCl3CHO+HClO��CCl3COOH+HCl���ϳɸ��л����ʵ��װ��ʾ��ͼ������װ��δ���������й��������£�
���� | C2H5OH | CCl3CHO | CCl3COOH | C2H5Cl |
�۵�/�� | -114.1 | -57.5 | 58 | -138.7 |
�е�/�� | 78.3 | 97.8 | 198 | 12.3 |
�ܽ��� | ��ˮ���� | ������ˮ���Ҵ� | ������ˮ���Ҵ� | ����ˮ���������Ҵ� |

��1����ѹ©��A��������________��Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ________��
��2��װ��B��������________��װ��F����������ʱ��Ϊʲô���Է�ֹҺ�巢����������________��
��3��װ��E�е��¶ȼ�Ҫ������70 ����������ƿ���õ���Ѽ��ȷ�ʽ��______�����Ҫ��������������ע����ˮ��������Ч������ˮӦ�ô�________���a����b������ͨ�롣ʵ��ʹ�����������ܶ���ʹ��ֱ�������ܵ�Ŀ����_______��
��4��ʵ����װ��C�е��Լ��DZ���ʳ��ˮ��װ����D���Լ���ŨH2SO4�������ʹ��Dװ�ã���Ʒ�л���ڽ϶������________���ѧʽ������ȥ��Щ���������ʵ�鷽����_______��
��5�����õ������ɲⶨ��Ʒ�Ĵ��ȣ���Ӧԭ�����£�
CCl3CHO+NaOH=CHCl3+HCOONa HCOONa+I2=HI+NaI+CO2�� I2+2Na2S2O3=2NaI+Na2S4O6
��ȡ��ʵ���Ʊ��IJ�Ʒ5.00 g�����100.00 mL��Һ��ȡ����10.00 mL��������ҺΪ���ʵ�pH����30.00 mL 0.100 mol��L1�ĵ��Һ����0.100 mol��L1��Na2S2O3��Һ�ζ����ظ�����3�β���������Na2S2O3��Һƽ�����Ϊ20.00 mL����ô�ʵ�����ò�Ʒ����Ϊ________��

