��Ŀ����
4��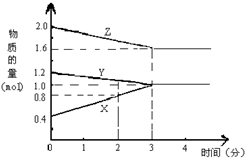 ij�¶�ʱ����2L�ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ�����
ij�¶�ʱ����2L�ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ�������1���÷�Ӧ�Ļ�ѧ����ʽ��Y+2Z?3X��
��2���÷�Ӧ��ƽ��ʱY��Ũ�ȣ�0.5mol/L��
��3����Ӧ��ʼ��2minĩ��Z�ķ�Ӧ����Ϊ0.1mol/��L•min����
��4�����з����п���֤���������淴Ӧ�Ѵ�ƽ��״̬����A��
���¶Ⱥ����һ��ʱ��X������������ٱ仯
���¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯��
���¶Ⱥ����һ��ʱ�����������ܶȲ��ٱ仯��
���¶�һ��ʱ��������ƽ����Է����������ٱ仯��
���� ��1�����ݲμӷ�Ӧ�����ʵ����ʵ���֮�ȵ��ڻ�ѧ������֮����д��ѧ����ʽ��
��2������ֵ����ʵ��������״̬Ϊƽ��״̬������3����ʱ��ƽ��״̬����ʱY�����ʵ���Ϊ1mol������c=$\frac{n}{V}$���㣻
��3����֪2minĩx�����ʵ���������v=$\frac{��c}{��t}$����x�����ʣ�����֮�ȵ��ڶ�Ӧ���ʵĻ�ѧ������֮�ȣ�
��4�����¶Ⱥ����һ��ʱ��X������������ٱ仯��˵�����淴Ӧ������ȣ�
���¶Ⱥ����һ��ʱ����Ϊ��������ļ�������ȣ�����������ѹǿʼ�ղ��仯��
���¶Ⱥ����һ��ʱ�����������ܶ�ʼ�ղ��䣻
����Ϊ���ߵļ�������ȣ������¶�һ��ʱ��������ƽ����Է�������һֱ���䣮
��� �⣺��1����ͼ����Կ�������Ӧ��Z��Y�����ʵ������٣�ӦΪ��Ӧ�X�����ʵ������࣬ӦΪ���������Ӧ���е�3minʱ����n��Y��=0.2mol����n��Z��=0.4mol����n��X��=0.6mol�����n��Y������n��Z������n��X��=1��2��3���μӷ�Ӧ�����ʵ����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ���Ӧ�ķ���ʽΪ��Y+2Z?3X��
�ʴ�Ϊ��Y+2Z?3X��
��2������ֵ����ʵ��������״̬Ϊƽ��״̬������3����ʱ��ƽ��״̬����ʱY�����ʵ���Ϊ1mol������c=$\frac{n}{V}$=$\frac{1mol}{2L}$=0.5mol/L���ʴ�Ϊ��0.5mol/L��
��3����Ӧ��ʼ��2minĩ��X�ķ�Ӧ����Ϊ��v=$\frac{��c}{��t}$=$\frac{\frac{0.4mol}{3L}}{2min}$=0.067mol/��L•min��������V��z��=$\frac{2}{3}$V��X��=$\frac{3}{2}��0.067mol/��L•min��$=0.1mol/��L•min����
�ʴ�Ϊ��0.1mol/��L•min����
��4�����¶Ⱥ����һ��ʱ��X������������ٱ仯��˵�����淴Ӧ������ȣ���ƽ��״̬������ȷ��
���¶Ⱥ����һ��ʱ����Ϊ��������ļ�������ȣ�����������ѹǿʼ�ղ��仯�����Բ�����ƽ��״̬�ı�־���ʴ���
���¶Ⱥ����һ��ʱ�����������ܶ�ʼ�ղ��䣬���Բ�����ƽ��״̬�ı�־���ʴ���
����Ϊ���ߵļ�������ȣ������¶�һ��ʱ��������ƽ����Է�������һֱ���䣬���Բ�����ƽ��״̬�ı�־���ʴ���
��ѡA��
���� ���⿼�黯ѧƽ��ͼ�����⣬��Ŀ�ѶȲ���ע�⻯ѧ����ʽ��ȷ���Լ���ѧƽ��״̬��������

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�| A�� | С�մ��������θ����࣬��������С�մ������ | |
| B�� | ��������Ũ��������Һ��Ӧ�����ʣ�NaHCO3��Na2CO3 | |
| C�� | �����ڿ����е��������̼���ƾ��� | |
| D�� | ijδ֪��Ʒ����ʱ��ʹ����ʻ�ɫ��˵������Ʒ�п϶�������Ԫ�� |
 | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | A | |||||||
| 2 | D | E | G | |||||
| 3 | B | C | J | F | H | I |
��2������������ˮ�����м�����ǿ����NaOH��������ǿ����HClO4��
��3��A�ֱ���D��E��F��G��H�γɵĻ������У����ȶ���HF��
��4����B��C��D��J��E��F��G��H�У�ԭ�Ӱ뾶������Na��
��5��д��D���⻯��ĵ���ʽ
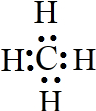 ��
����6��E��H���⻯�����Ӧ��������ɫ���壬�ù����д��ڵĻ�ѧ�����������Ӽ������ۼ���
��7���õ���ʽ��������BH���γɹ��̣�
 ��
�� 
| A�� | X����ΪCO2 | |
| B�� | ������ĺ��������ˮpH���� | |
| C�� | �м����е�Cl-�������� | |
| D�� | ��·��ÿͨ��1mol���ӣ�������״���µ��������Ϊ2.24L |
| A�� | �ԷϾɵ�ؽ��л��մ��� | |
| B�� | ����ʱ�����������ϴ� | |
| C�� | �Ծ���ϩ��������������������㵹�뺣 | |
| D�� | ʹ��������̫���ܡ����ܵ���Դ�����ͳ��ú̿ |
| A�� | H2O | B�� | CH3COOC2H5 | C�� | CH3CH2OH | D�� | CH3COOH |
 A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ�
A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ� ��
�� ��
�� �������ף�POCl3��������Ϊ��ɫҺ�壬�й㷺Ӧ�ã����������������Ĺ�ҵ���������Ȼ��ġ�����ֱ�������������洫ͳ�����Ȼ��ס��Ȼ�ˮ�ⷨ���������������Ȼ���ˮΪԭ�Ϸ�Ӧ�õ�����
�������ף�POCl3��������Ϊ��ɫҺ�壬�й㷺Ӧ�ã����������������Ĺ�ҵ���������Ȼ��ġ�����ֱ�������������洫ͳ�����Ȼ��ס��Ȼ�ˮ�ⷨ���������������Ȼ���ˮΪԭ�Ϸ�Ӧ�õ����� AgSCN��S��+Cl-��aq�����÷�Ӧʹ�ⶨ���ƫ�ͣ�����ƫ�ߣ�ƫ�ͻ䣩
AgSCN��S��+Cl-��aq�����÷�Ӧʹ�ⶨ���ƫ�ͣ�����ƫ�ߣ�ƫ�ͻ䣩