��Ŀ����
15��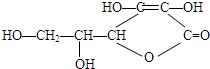 �л�������Ļ�ѧ���ʱ����Ͼ��Ƿ��ӽṹ�����������ŵ����ʣ�
�л�������Ļ�ѧ���ʱ����Ͼ��Ƿ��ӽṹ�����������ŵ����ʣ���1����-CH3��-OH��-H��-COOH��-C6H5���ֻ����е����ֲ�ͬ�������ϣ����γɵ��л�����ֻ�������Ե������У�CH3COOH��HCOOH��C6H5COOH��C6H5OH��д����Ӧ���ʵĽṹ��ʽ����
��2��ά����C�Ľṹ��ʽ��ͼ����
��ά����C�ķ���ʽΪ��C6H8O6��
��1molά����C������4mol�����Ʒ�Ӧ��
��ά����C������������ˮ�⣬������Ľṹ��ʽ��
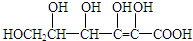 ��
�������й���ά����C��������������ȷ����cd��
a������������Ӧ�����������ܷ���������Ӧ
b��������ˮ�����ӳɷ�Ӧ
c��ά����C������ˮ
d���ڼ��������¿��ȶ����ڣ�
���� ��1����-H��-CH3�� ��-OH��-COOH����������γɵ��л���������ԣ������DZ������ǻ�ֱ��������Ҳ����Ϊ�Ȼ������������γ����ᣬע��Ϊ�л����Ҫ��
��-OH��-COOH����������γɵ��л���������ԣ������DZ������ǻ�ֱ��������Ҳ����Ϊ�Ȼ������������γ����ᣬע��Ϊ�л����Ҫ��
��2�������л���Ľṹ��ʽ��ȷ������ʽ�����л����к���-OH��-O-��C=O�Լ�C=C�����л�ԭ�ԣ��������Ը��������Һ��Ӧ����ʹʯ����Һ��Һ�Ժ�ɫ��Ӧ�������ԣ�
��� �⣺��1��������ͷӾ������ԣ���-H��-CH3��-OH��-COOH�� �������ɵľ������Ե��л���Ϊ��HCOOH��CH3COOH��C6H5COOH��C6H5OH��
�������ɵľ������Ե��л���Ϊ��HCOOH��CH3COOH��C6H5COOH��C6H5OH��
�ʴ�Ϊ��CH3COOH��HCOOH��C6H5COOH��C6H5OH��
��2���ٸ��ݽṹ��ʽ��֪�����к���6��C��8��H��6��O�������ʽΪC6H8O6���ʴ�Ϊ��C6H8O6��
�ڷ����к���4���ǻ�����1molά����C������4mol�����Ʒ�Ӧ���ʴ�Ϊ��4��
�ۺ�����������ˮ�������Ȼ����ǻ���ˮ�����Ϊ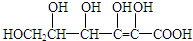 ���ʴ�Ϊ��
���ʴ�Ϊ��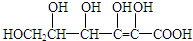 ��
��
��a������-CH2OH�ṹ���ɷ���������Ӧ����ȩ���ܷ���������Ӧ����a��ȷ��
b������̼̼˫����������ˮ�����ӳɷ�Ӧ����b��ȷ��
c�����ж���ǻ���ά����C������ˮ����c����
d�������������ڼ���������ˮ�⣬��d����
�ʴ�Ϊ��cd��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬��Ŀ�ѶȲ�����ע������л���Ĺ����ŵ���������ʣ�����ʱע�������Ϣ��

| A�� | NH4Cl | B�� | ��NH4��2CO3 | C�� | NaCl | D�� | K2SO4 |
| A�� | �������ܡ�̫���ܡ����ܵ�����Դ��ʹ������ϴ�Ӽ�����ֱ�ӽ���̼�ŷ� | |
| B�� | �������������DZ�������CO2������ת��ɻ�ѧ�ܵĹ��� | |
| C�� | ��ʯȼ�Ϻ�ֲ��ȼ��ȼ��ʱ�ų�����������Դ��̫���� | |
| D�� | ��Խ��ܼ�����Ҫ���������������վ |
| A�� | Al2O3��Al��OH��3 | B�� | CaCO3��Ca��OH��2 | C�� | CH3CH2OH��CH3CHO | D�� | CO��Na2CO3 |
| A�� | ��������1.4g | B�� | ������ҺpH��1 | C�� | ��������0.64g | D�� | ������ҺpH��1 |
 ]-��
]-��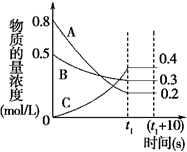 ��һ�������£�A������B���巴Ӧ����C���壮��Ӧ�����У���Ӧ�����������Ũ����ʱ��仯��������ͼ���ش��������⣺
��һ�������£�A������B���巴Ӧ����C���壮��Ӧ�����У���Ӧ�����������Ũ����ʱ��仯��������ͼ���ش��������⣺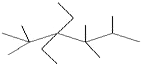 ������Ϊ2��2��4��4��5-���-3��3-���һ����飮
������Ϊ2��2��4��4��5-���-3��3-���һ����飮