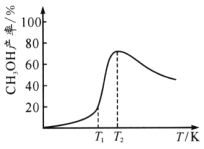
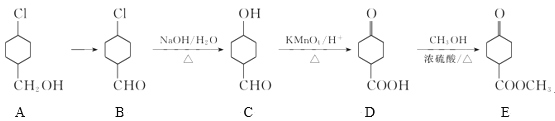
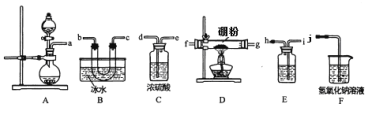
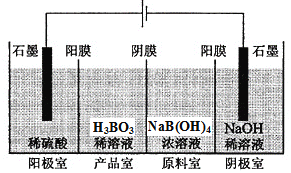
��Ŀ����
����Ŀ����1���ֽ�200mL0.30mol/L��������50mL0.80mol/LCaCl2��Һ���(��Ϻ�����仯���Բ���)��������Һ��Cl�������ʵ���Ũ����___��
��2����20.0g�������ƹ�������ˮ���100mL��Һ�����ܶ�Ϊ1.25g��mL��1������Һ���������Ƶ����ʵ���Ũ��Ϊ___����������Ϊ___���Ӹ���Һ��ȡ��10 mL�������ˮϡ�͵�100 mL��ϡ�ͺ���Һ���������Ƶ����ʵ���Ũ��Ϊ___����һ�������ԭ��Һ��ϡ�ͺ����Һ��1��4������Ȼ�ϣ����Ի��ʱ��Һ����仯�������û����Һ���������Ƶ����ʵ���Ũ��Ϊ___��
��3�����廯����A����ʽ�ɱ�ʾΪOxFy����֪ͬ��ͬѹ��10mLA���ȷֽ�����15mLO2��10mLF2����A�Ļ�ѧʽΪ___���ƶϵ�����Ϊ___��
���𰸡�0.56mol/L 5mol/L 16% 0.5mol/L 1.4mol/L O3F2 ͬ��ͬѹ�£����������ȵ������ʵ���֮���ֵ��ڻ�ѧ����ʽ������֮��
��������
��1����200mL0.30mol/L��������50mL0.80mol/LCaCl2��Һ���(��Ϻ�����仯���Բ���)��������Һ��Cl�������ʵ���Ũ����![]() ����Ϊ��0.56mol/L��
������0.56mol/L��
��2����20.0g�������ƹ�������ˮ���100mL��Һ�����ܶ�Ϊ1.25g��mL��1������Һ���������Ƶ����ʵ���Ũ��Ϊ ����Ϊ��5mol/L��
������5mol/L��
��������Ϊ![]() ����Ϊ��16%��
������16%��
�Ӹ���Һ��ȡ��10 mL�������ˮϡ�͵�100 mL��ϡ�ͺ���Һ���������Ƶ����ʵ���Ũ��Ϊ![]() ����Ϊ��0.5mol/L��
������0.5mol/L��
��һ�������ԭ��Һ��ϡ�ͺ����Һ��1��4������Ȼ�ϣ����Ի��ʱ��Һ����仯�������û����Һ���������Ƶ����ʵ���Ũ��Ϊ![]() ����Ϊ��1.4mol/L��
������1.4mol/L��
��3�����廯����A����ʽ�ɱ�ʾΪOxFy����֪ͬ��ͬѹ��10mLA���ȷֽ�����15mLO2��10mLF2��
2OxFy = xO2 + yF2
2mL xmL ymL
10mL 15mL 10mL
��ã�x=3 ��y=2
��A�Ļ�ѧʽΪO3F2����Ϊ��O3F2��
�ƶϵ�����Ϊ��ͬ��ͬѹ�£����������ȵ������ʵ���֮���ֵ��ڻ�ѧ����ʽ������֮�ȡ�
��Ϊ��ͬ��ͬѹ�£����������ȵ������ʵ���֮���ֵ��ڻ�ѧ����ʽ������֮�ȡ�