��Ŀ����
20����֪��������ͨ����������S8��б������ʽ���ڣ���������״̬ʱ������S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ��ͼ1��ʾ����1����һ���¶��£������������ƽ��Ħ������Ϊ96g/mol�����������S2���ӵ����������С��50%��
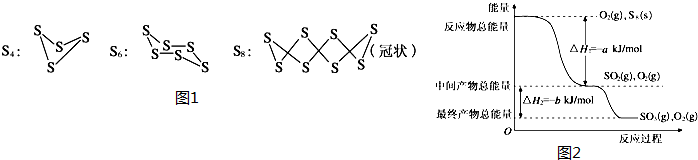
��2����һ�������£�S8��s����O2��g��������Ӧ����ת��ΪSO2��g����SO3��g������Ӧ���̺�������ϵ����ͼ2��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
��д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽS8��s��+8O2��g���T8SO2��g����H=-8akJ•mol-1��
������֪SO2�������������ļ���ΪdkJ•mol-1��O=O���ļ���ΪekJ•mol-1����S8������S-S���ļ���Ϊ��2d-a-e��kJ•mol-1��
���� ��1����S2��S4 ʱS2���ӵ����������С�����ƽ��ֵ�����������������
��2����S8��s����O2��g��������Ӧת��ΪSO2��g��ʱ����Ϊȼ���ȣ���ע���ʾۼ�״̬�ͷ�Ӧ�ʱ�д���Ȼ�ѧ����ʽ��
��SO2�������������ļ���ΪdkJ•mol-1��O=O���ļ���ΪekJ•mol-1����Ϸ�Ӧ�ȵ��ڷ�Ӧ��ļ����ܺͼ�ȥ����������ܺͼ��㣮
��� �⣺��1����S2��S4ʱS2���ӵ����������С����S2���ʵ���Ϊx��S4���ʵ���Ϊy����$\frac{64x+128y}{x+y}$=96�����x��y=1��1����ͬ�������������ʵ���֮�ȵ������֮����õ�����������S2���ӵ����������С��$\frac{1}{1+1}$��100%=50%���ʴ�Ϊ��50%��
��2����S8��s����O2��g��������Ӧת��ΪSO2��g��ʱ����Ϊȼ���ȣ���ͼ��֪����1molSO2��g���ų�����ΪakJ����ȼ���ȵ�S8��s��+8O2��g���T8SO2��g����H=-8akJ•mol-1���ʴ�Ϊ��S8��s��+8O2��g���T8SO2��g����H=-8akJ•mol-1��
����֪��������S=O���ļ���Ϊd kJ/mol����������O=O���ļ���Ϊe kJ/mol������S8���������������ΪxKJ/mol����S8��s��+8O2��g��=8SO2��g����H=-8aKJ/mol����Ӧ��=��Ӧ��ļ����ܺ�-������ļ����ܺͿ�֪��8x+8e-16d=-8a�����x=2d-a-e��
�ʴ�Ϊ����2d-a-e��KJ/mol��
���� �����Ժ������ʿ����Ȼ�ѧ��Ӧ�����㣬Ϊ��Ƶ���㣬����ͼ��ȼ���ȵĸ����Ӧ������ܵĹ�ϵ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

��SeO2+4KI+4HNO3=Se+2I2+4KNO3+2H2O����Se+2H2SO4��Ũ��=2SO2��+SeO2+2H2O��
| A�� | ����Se���������I2�ǻ�ԭ���� | |
| B�� | SeO2��H2SO4��Ũ����I2����������ǿ������˳���� H2SO4��Ũ����SeO2��I2 | |
| C�� | ���³�ѹ�£�Se�ǹ��壬������������Ӧ��ˮ������H2SeO4 | |
| D�� | ��Ӧ����ÿ��0.6 mol I2���ɣ�ת�Ƶ�����ĿΪ2.4NA |
| A�� | ����������Ȼ�л��߷��ӻ����� | B�� | ��I-�ܱ���ɫ | ||
| C�� | ���۲�������ˮ | D�� | ��������������ˮ������������ |
| A�� | �ڻ������г�+2�� | B�� | ��������ˮ��Ӧ�ų�H2 | ||
| C�� | ������������Ϊǿ�� | D�� | ��Ԫ�صĽ����Աȱ�Ԫ�ص��� |
| A�� | 0.1 mol•L-1•min-1 | B�� | 0.2 mol•L-1•min-1 | ||
| C�� | 0.3 mol•L-1•min-1 | D�� | 0.6 mol•L-1•min-1 |
| A�� | Na2CO3�T2Na++CO32- | B�� | NaHSO4�TNa++H++SO42- | ||
| C�� | NaHCO3�TNa++H++CO32- | D�� | KClO3�TK++Cl-+3O2- |
| A�� | H2O | B�� | HCl | C�� | MgCl2 | D�� | SO2 |
| A�� | ������ȼ����Ϊ285.8 kJ/mol����ˮ�����Ȼ�ѧ����ʽΪ��2H2O��l��=2H2��g��+O2��g����H=+285.8 kJ/mol | |
| B�� | 1 mol������ȫȼ������CO2��H2O��l��ʱ�ų�890kJ������������ȼ���Ȼ�ѧ����ʽΪ��$\frac{1}{2}$CH4��g��+O2��g��=$\frac{1}{2}$CO2 ��g��+H2O��l����H=-445 kJ/mol | |
| C�� | HF��NaOH��Һ��Ӧ��H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ/mol | |
| D�� | ��ʯī�Ƚ��ʯ�ȶ���֪��C�����ʯ��s��=C��ʯī��s����H��0 |
 ��B��CH��CH��C��CH2�TCH2��D��CH3CH3��
��B��CH��CH��C��CH2�TCH2��D��CH3CH3�� ��
��