��Ŀ����
����Ŀ��ij��ѧ����С���о���������������ʱ���ı�ijһ������A2��g��+3B2��g��![]() 2AB2��g����ѧƽ��״̬��Ӱ�죬�õ�����ͼ��ʾ�ı仯���ɣ�ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ��������ݴ˿ɵó����жϽ��۲���ȷ���� �� ��
2AB2��g����ѧƽ��״̬��Ӱ�죬�õ�����ͼ��ʾ�ı仯���ɣ�ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ��������ݴ˿ɵó����жϽ��۲���ȷ���� �� ��
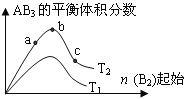
A. �ﵽƽ��ʱA2��ת���ʴ�СΪ��b>a
B. a��b��c�����ƽ�ⳣ����ͬ
C. ��T2>T1��������Ӧһ�������ȷ�Ӧ
D. b��ʱ��ƽ����ϵ��A��Bԭ����֮��һ����1��1
���𰸡�D
��������
A����ͼ��֪��������ΪB2�����ʵ���������һ�ַ�Ӧ�������Ȼ��ٽ���һ�ַ�Ӧ���ת������B2Խ��ﵽƽ��ʱA2��ת����Խ���ﵽƽ��ʱA2��ת���ʴ�СΪb��a����A��ȷ��
B��a��b��c����Ϊͬһ�¶��µ�ƽ�⣬�¶���ͬ��ƽ�ⳣ����ͬ������a��b��c�����ƽ�ⳣ����ͬ����B��ȷ��
C����ͼ��֪��B2��ʼ��ͬʱ��T2��Ӧ��AB3�ĺ���������������ӦΪ���ȷ�Ӧ����T2��T1����������ӦΪ���ȷ�Ӧ����T2��T1����C��ȷ��
D��b��AB3��������������Ӧ������ʵ���֮�Ƚӽ����ڻ�ѧ������֮�ȣ���Ӧ���Ϊ˫ԭ�ӷ��ӣ�����ƽ����ϵ��A��Bԭ����֮�Ƚӽ�1��3����D����
��ѡD��

����Ŀ����֪��ѧ��Ӧ�٣�Fe(s)��CO2(g)![]() FeO(s)��CO(g)����ƽ�ⳣ��ΪK1����ѧ��Ӧ�ڣ�Fe(s)��H2O(g)
FeO(s)��CO(g)����ƽ�ⳣ��ΪK1����ѧ��Ӧ�ڣ�Fe(s)��H2O(g)![]() FeO(s)��H2(g)����ƽ�ⳣ��ΪK2����ѧ��Ӧ��CO2(g)��H2(g)
FeO(s)��H2(g)����ƽ�ⳣ��ΪK2����ѧ��Ӧ��CO2(g)��H2(g)![]() H2O(g)��CO(g)����ƽ�ⳣ��ΪK3,���¶�Ϊ973K��1173K����£�K1��K2��ֵ�ֱ����£�
H2O(g)��CO(g)����ƽ�ⳣ��ΪK3,���¶�Ϊ973K��1173K����£�K1��K2��ֵ�ֱ����£�
�¶� | K1 | K2 |
973 K | 1.47 | 2.38 |
1173 K | 2.15 | 1.67 |
����գ�
��1��ͨ�������е���ֵ�����ƶϣ���Ӧ����____(����ȡ����ȡ�)��Ӧ��
��2�����ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵʽ_____���ݴ˹�ϵʽ���ϱ����ݣ�Ҳ���ƶϳ���Ӧ����____(����ȡ����ȡ�)��Ӧ��
��3��Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ�Ĵ�ʩ��____��
A����С��Ӧ�������ݻ�
B������Ӧ�������ݻ�
C�������¶�
D��ʹ�ú��ʵĴ���
E���跨��Сƽ����ϵ��CO��Ũ��
��4��ͼ�ס�ͼ�ҷֱ��ʾ��Ӧ����t1ʱ�̴ﵽƽ�⣬��t2ʱ����ı�ij�������������仯�������

��ͼ��t2ʱ�̸ı��������___����ͼ��t2ʱ�̸ı��������__��
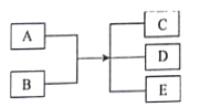
����Ŀ���±����и��������У�����֮��ͨ��һ����Ӧ��ʵ����ͼ��ʾת������( )
X | Y | Z | ����ת����ϵ | |
A | Cu | CuO | Cu(OH)2 |
|
B | Si | SiO2 | H2SiO3 | |
C | NaHCO3 | Na2CO3 | NaOH | |
D | FeCl2 | FeO | FeCl3 |
A. A B. B C. C D. D