��Ŀ����
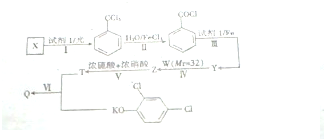
18������ϩ��Z�Ǿ��й㷺��;�ĺϳɸ߷��Ӳ��ϣ��ṹ��ʽΪ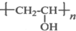 ���乤ҵ�ϳ�·�����£�
���乤ҵ�ϳ�·�����£�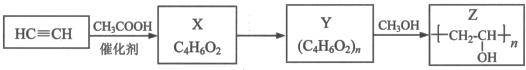
��֪�����з�Ӧ��R��R�䣬R���������
i��RCOOH+HC��CH$\stackrel{����}{��}$RCOOOCH=CH2
ii��RCOOR��+R��OH��RCOOR��+R��OH
�ش��������⣺
��1��X�Ľṹ��ʽ��CH3COOCH=CH2��
��2��Yת��ΪZ�Ļ�ѧ����ʽ��
 ��
����3����֪��

�ں������������ʡ��ڷ���������ϵ����ɾ���ϩ��ת��Ϊά�ڵĻ�ѧ����ʽ��
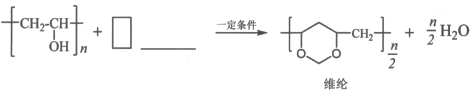
���� ��X����ʽ�������Ϣi����֪X�ṹ��ʽΪCH3COOCH=CH2��X�����Ӿ۷�Ӧ�õ�YΪ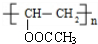 ������Ϣii��֪��Y��״������Ӿ۷�Ӧ����
������Ϣii��֪��Y��״������Ӿ۷�Ӧ����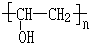 ��ͬʱ������������ɣ�
��ͬʱ������������ɣ�
��1����2����X����ʽ�������Ϣi����֪X�ṹ��ʽΪCH3COOCH=CH2��X�����Ӿ۷�Ӧ�õ�YΪ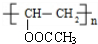 ������Ϣii��֪��Y��״������Ӿ۷�Ӧ����
������Ϣii��֪��Y��״������Ӿ۷�Ӧ����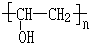 ��ͬʱ������������ɣ�
��ͬʱ������������ɣ�
��3������Ŀ��Ϣ����Ͼ���ϩ����ά�ڵĽṹ����֪����ϩ����HCHO��Ӧ����ά�ڣ�����Cԭ���غ��֪��ȩ��ϵ��Ϊ$\frac{n}{2}$��
��� �⣺��X����ʽ�������Ϣi����֪X�ṹ��ʽΪCH3COOCH=CH2��X�����Ӿ۷�Ӧ�õ�YΪ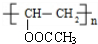 ������Ϣii��֪��Y��״������Ӿ۷�Ӧ����
������Ϣii��֪��Y��״������Ӿ۷�Ӧ���� ��ͬʱ������������ɣ�
��ͬʱ������������ɣ�
��1��������������֪��X�ṹ��ʽΪCH3COOCH=CH2���ʴ�Ϊ��CH3COOCH=CH2��
��2��Yת��ΪZ�Ļ�ѧ����ʽ�ǣ� ��
��
�ʴ�Ϊ�� ��
��
��3������Ŀ��Ϣ����Ͼ���ϩ����ά�ڵĽṹ����֪����ϩ����HCHO��Ӧ����ά�ڣ�����Cԭ���غ��֪��ȩ��ϵ��Ϊ$\frac{n}{2}$��
�ʴ�Ϊ��$\frac{n}{2}$��HCHO��
���� ���⿼���л���ϳ����ƶϣ���������л���Ľṹ�����ʽ����Ӧ��Ϣ���з�����𣬲��ؿ���ѧ������������֪ʶǨ��Ӧ�ã��Ѷ��еȣ�

| A�� | �ܶ�֮��1��2��3 | B�� | ������֮��6��3��2 | C�� | ������֮��0��3��4 | D�� | ���֮��6��3��2 |
| A�� | 2��3-�������� | B�� | 3��3-�������� | ||
| C�� | 2-��-3-�һ����� | D�� | 2��2��3-�������� |
| A�� | ��ϡ����μӵ�װ���۵��Թ��� | |
| B�� | ��NaOH��Һ��ͨ��SO2���� | |
| C�� | �ֱ�Cl2��SO2ͨ��ˮ�гɷַ�Ӧ�����Һ | |
| D�� | ��MgSO4��H2SO4�Ļ��Һ�м������Ba��OH��2��Һ |
| A�� | NH3 | B�� | H2O | C�� | HF | D�� | CH4 |
| A�� | ���ý�̿��һ����̼��ԭ�������ķ�����ұ���� | |
| B�� | �õ��NaCl��Һ�ķ�����ұ�������� | |
| C�� | ��Щ���ý��������������Ȼ�ԭ���Ļ�ԭ�� | |
| D�� | �����ڵؿ��к����ܸߣ����շϾɽ����˷��������� |
| A�� | ȼ�ղ���ֻ��CO2��H2O | B�� | ��±�ص��ʷ���ȡ����Ӧ | ||
| C�� | ������ˮ | D�� | ͨ������ǿ�ᡢǿ�ǿ��������Ӧ |
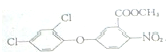

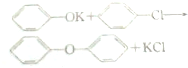
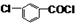 +CH3OH��
+CH3OH�� +HCl��
+HCl�� ��
��