��Ŀ����
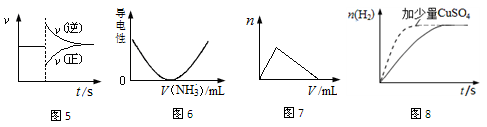
��10�֣������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����

�ش��������⣺
��1��������Ӧ���������������� (����)��
��2����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+,��̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ:
�������������������������������������� ��������������������������������������
��Һ��������������������������������������
��3����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ ��

�ش��������⣺
��1��������Ӧ���������������� (����)��
��2����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+,��̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ:
�������������������������������������� ��������������������������������������
��Һ��������������������������������������
��3����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ ��
(1) 4 8
(2 )2H++2e-=H2�� Fe-2e-=Fe2+ Cr2O72- + 6Fe2+ + 14H+ = 2Cr3+ +6Fe3++7H2O
(3)2CrO42-+2H+ Cr2O72-+H2O
Cr2O72-+H2O
(2 )2H++2e-=H2�� Fe-2e-=Fe2+ Cr2O72- + 6Fe2+ + 14H+ = 2Cr3+ +6Fe3++7H2O
(3)2CrO42-+2H+
 Cr2O72-+H2O
Cr2O72-+H2O���⿼����������ԭ��Ӧ�����ӷ�Ӧ���绯ѧ�����֪ʶ��
��1�����ݸ��Ļ��ϼۿ�֪�����ϼ����ߵķ�Ӧ��Ȼ��������ṩ������
��2��������Ҫʧ���� Fe-2e-=Fe2+���ٸ������ӵķŵ�˳��֪��H+�������ŵ�2H++2e-=H2�� ��
Fe2+�IJ����ṩ�˻�ԭ������Cr2O72-������Cr2O72- + 6Fe2+ + 14H+ = 2Cr3+ +6Fe3++7H2O
��3����Ҫ�������ӷ���ʽ����д����ƽ
��1�����ݸ��Ļ��ϼۿ�֪�����ϼ����ߵķ�Ӧ��Ȼ��������ṩ������
��2��������Ҫʧ���� Fe-2e-=Fe2+���ٸ������ӵķŵ�˳��֪��H+�������ŵ�2H++2e-=H2�� ��
Fe2+�IJ����ṩ�˻�ԭ������Cr2O72-������Cr2O72- + 6Fe2+ + 14H+ = 2Cr3+ +6Fe3++7H2O
��3����Ҫ�������ӷ���ʽ����д����ƽ

��ϰ��ϵ�д�
 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
�����Ŀ
 CH3OH(g) ��H1����116 kJ��mol-1
CH3OH(g) ��H1����116 kJ��mol-1

 CH3CH2OH(g)+H2O(g) ��H=��256.1kJ��mol��1��
CH3CH2OH(g)+H2O(g) ��H=��256.1kJ��mol��1�� CO2(g)+H2(g) ��H=��41.2kJ��mol��1
CO2(g)+H2(g) ��H=��41.2kJ��mol��1
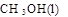 +
+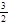
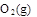 =
= 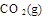 + 2
+ 2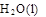 ��H = ��725.5 kJ��mol��1
��H = ��725.5 kJ��mol��1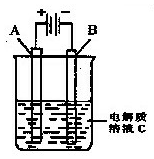
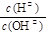 = 1��10�� 6 �������Һ��pHΪ____����Һ�е����ʵ������������Ũ��ԼΪ ���� pH = 4��������ҺV1 L�� 0.01 mol��L��1��ˮV2 L��ϣ��������ҺpH = 7����V1��V2�Ĺ�ϵΪ��V1 V2���>������<����=������
= 1��10�� 6 �������Һ��pHΪ____����Һ�е����ʵ������������Ũ��ԼΪ ���� pH = 4��������ҺV1 L�� 0.01 mol��L��1��ˮV2 L��ϣ��������ҺpH = 7����V1��V2�Ĺ�ϵΪ��V1 V2���>������<����=������ CH3OH(g)
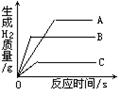
CH3OH(g) �����������仯����ͼ��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽ__________________��
�����������仯����ͼ��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽ__________________��
 ��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��
��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��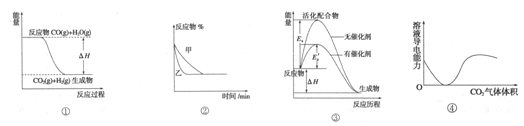
 5C02 (g)+H2 (g)��H>0
5C02 (g)+H2 (g)��H>0