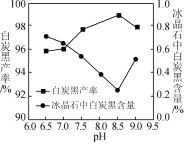
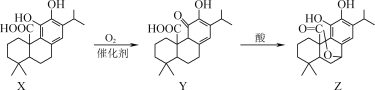
��Ŀ����
����Ŀ��I.��VIA��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
��1��S���ʵij�����ʽΪS8���价״�ṹ��ͼ1��ʾ��Sԭ�Ӳ��õĹ���ӻ���ʽ��_____��

��2��H2SeO4��H2SeO3����ǿ��ԭ��_________��
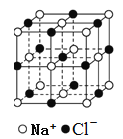
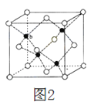
��3��ZnS�ڹ����ϡ�ӫ���塢Ϳ�ϡ����ϵ���ҵ��Ӧ�ù㷺������ZnS����ṹ��ͼ2��ʾ���侧���߳�Ϊa pm���ܶ�Ϊ______gcm3��(�ú�a��NA�ı���ʽ���)
II�������仯��������������������ϢϢ��ء�����(NH4)2SO4�лẬ��N4H4(SO4)2����������ˮ�е����SO42-��N4H44+��N4H44+����������Һ������һ�����ư���(P4)��N4���ӡ���ش��������⣺
��4��N4�����еĵ������ļ���Ϊ____��1molN4�����к��еĵ���������ĿΪ____��
��5��N4��P4�ķе�____����������������������ԭ��Ϊ________��
���𰸡�sp3 ���ǻ���ԭ�Ӷ� ![]() 60�b 6NA �� N4��P4���ǷǼ��Է��ӣ�N4��P4����Է�������С�����Ӽ䷶�»�����
60�b 6NA �� N4��P4���ǷǼ��Է��ӣ�N4��P4����Է�������С�����Ӽ䷶�»�����
��������
����ͼʾ�Ŀռ�ṹ�жϻ�ѧ�������ͼ�����ӻ���ʽ�����þ�̯��ȷ����ѧʽ�����ݾ����Ľṹ���㾧�����ܶȣ����ݾ������ͼ����ӵļ��ԱȽ����ʵ��۷е㡣
I.��1����ͼ��ʾ��ÿ��S�γ������Ҽ�������2�Թ¶Ե��ӣ��۲���Ӷ���Ϊ4����Sԭ�Ӳ��õĹ���ӻ���ʽ��sp3��
��2��H2SeO3�ķ����е�SeΪ+4�ۣ����ǻ���ԭ����Ϊ1����H2SeO4�ķ����е�SeΪ+6�ۣ����ǻ���ԭ����Ϊ2������Seԭ������������ǿ�������ǻ�����ԭ�Ӹ��������H+����������H2SeO4��H2SeO3����ǿ��ԭ��Ϊ����������з��ǻ���ԭ�ӽ϶ࣻ
��3�����þ�̯�����㾧����Sԭ����ĿΪ8��1/8+6��1/2=4��Znԭ����ĿΪ4�����ܶ�Ϊ��![]() ��
��
II����4��N4�����ڰ��Ľṹ��������Ϊ��������ṹ������N4�����еĵ������ļ���Ϊ60�b��1molN4�����к��еĵ���������ĿΪ6NA��
��5��N4��P4�ķе�ͣ�ԭ��ΪN4��P4���ǷǼ��Է��ӣ�N4��P4����Է�������С�����Ӽ䷶�»�������

 ������ȫ��������ϵ�д�
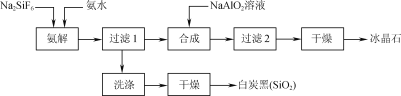
������ȫ��������ϵ�д�����Ŀ��ʵ�������̷�(FeSO4��7H2O)Ϊԭ���Ʊ���Ѫ���ʰ�������[(H2NCH2COO)2Fe]���й�������������:
�ʰ���(H2NCH2COOH) | ������ | �ʰ������� |
������ˮ�������Ҵ������Ի����� | ������ˮ���Ҵ��������Ժͻ�ԭ�� | ������ˮ���������Ҵ� |
ʵ�����:
I.���ƺ�0.10molFeSO4���̷���Һ��
II.�Ʊ�FeCO3:�����ƺõ��̷���Һ�У���������200mL1.1mol/LNH4HCO3��Һ���ӱ߽��裬��Ӧ��������˲�ϴ�ӳ�����
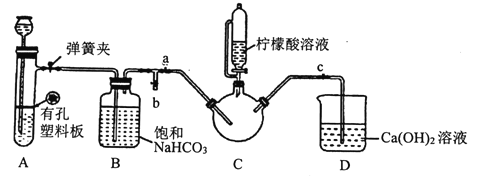
III.�Ʊ�(H2NCH2COO)2Fe:ʵ��װ������ͼ(�гֺͼ���������ʡ��)����ʵ��II�õ��ij����ͺ�0.20mol�ʰ����ˮ��Һ��Ϻ����C�У�Ȼ������A�з�Ӧ���������彫C�п����ž������ŵ�����������Һ�����ȡ���Ӧ��������ˣ���Һ�������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
�ش���������:
(1)ʵ��I��:ʵ���������̷���Һʱ��Ϊ��ֹFeSO4���������ʣ�Ӧ������Լ�Ϊ____(д��ѧʽ)��
(2)ʵ��II��:���ɳ��������ӷ���ʽΪ________________��
(3)ʵ��III��:
�ټ��װ��A�������Եķ�����_________��
��װ��A����ʢ�ŵ�ҩƷ��_______ (�����)��
a.Na2CO3��ϡH2SO4 b.CaCO3��ϡH2SO4 c.CaCO3��ϡ����
��ȷ��c�п����ž���ʵ��������______________��
�ܼ�����������Һһ����ɵ�����Һ��pH�ٽ�FeCO3�ܽ⣬��һ��������_______��
��ϴ��ʵ��III�еõ��ij�������ѡ�õ����ϴ���Լ���___(�����)��
a.��ˮ b.�Ҵ���Һ c.��������Һ
������Ʒ������Ϊ17.34g,�����Ϊ_____��