��Ŀ����
����Ŀ��ʹ���ʸ������������(Na2SiF6)Ϊԭ�Ϻϳɱ���ʯ(Na3AlF6)��Ϊһ������������Դ����߾���Ч����·�����������������ͼ��ʾ��
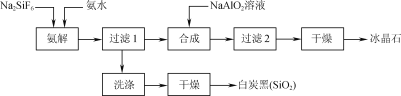
��1����ͳ�ϳɱ���ʯ�ķ�����өʯ(CaF2)����ʹ��өʯ��ʯӢ�ʹ����ڸ�������������NaF����NaF��Һ�м���Al2(SO4)3��Һ�Ƶá��ڼ�����������Һǰ�����������ὫNaF��Һ��pH�µ���5���ң�
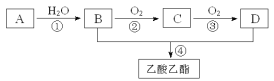
������ܲ���������____(�����ʵĻ�ѧʽ)������Ȳ��˹�ǿ��ԭ����____��
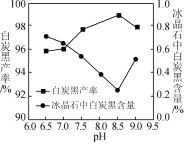
��2������ʱ��̿�ڲ��ʺͱ���ʯ������pH�Ĺ�ϵ��ͼ������ʱ��Ҫ������Һ��pH��____������߰������ʵĴ�ʩ��____(����ĸ)��
A�����ٽ���
B�����Ȼ��Һ��100��
C����С��ˮŨ��
��3�����������а��ⷴӦ�Ļ�ѧ����ʽΪ____������ʯ�ϳɷ�Ӧ�����ӷ���ʽΪ______��
��4��Ϊ�����ԭ�������ʣ����ٻ�����Ⱦ���ɲ�ȡ�Ĵ�ʩ��___��
���𰸡�Al(OH)3 H����F�������������HF��ʹF��Ũ�ȼ��Ͳ�������Na3AlF6 8.5 A Na2SiF6��4NH3��H2O=2NaF��4NH4F��SiO2����2H2O 3Na����4NH4+��6F����AlO2-��2H2O=Na3AlF6����4NH3��H2O ����2����Һ��ˮ��ѭ������
��������
��������ͼ����ˮ��ȡ��������ʱ�����˶������裬��ͬʱ������NaF��NH4F��H2O����Ӧ�ķ���ʽ���Ա�ʾΪNa2SiF6+4NH3H2O�T2NaF+4NH4F+ SiO2��+2H2O�������Һ�к���NaF��NH4F������NaAlO2��Һ�õ�Na3AlF6������2�õ�����ҺΪ����ˮ��Һ����ѭ��ʹ�ã��ݴ˷������
(1)��ͳ�ϳɱ���ʯ�ķ�����өʯ(CaF2)������ʹ��өʯ��ʯӢ�ʹ����ڸ�������������NaF����NaF��Һ�м���Al2(SO4)3��Һ�Ƶá�NaFˮ�⣬��Һ�Լ��ԣ��ڼ�����������Һǰ�����������ὫNaF��Һ��pH�µ���5���ң���ֹ����������������Al(OH)3����������Ȳ��˹�ǿ������H����F�������������HF��ʹF��Ũ�ȼ��Ͳ�������Na3AlF6���ʴ�Ϊ��Al(OH)3��H����F�������������HF��ʹF��Ũ�ȼ��Ͳ�������Na3AlF6��
(2)��ͼ��֪��pHΪ8.5ʱ������ʯ�а�̿�ڵĺ����ϵͣ�����ʯ���Ƚϸߣ���̿�ڲ��ʸߣ�A�����ٽ�����Լӿ췴Ӧ���ʣ���A��ȷ��B�����Ȼ��Һ��100������ˮ���ȷֽ⣬��Ӧ��Ũ��С����ѧ��Ӧ���ʼ�������B����C����С��ˮŨ�ȣ���ѧ��Ӧ���ʼ�������C���ʴ�Ϊ��8.5��A��
(3) ��������ͼ����ˮ��ȡ��������ʱ�����˶������裬��ͬʱ������NaF��NH4F��H2O����Ӧ�ķ���ʽ���Ա�ʾΪNa2SiF6+4NH3H2O�T2NaF+4NH4F+ SiO2��+2H2O��ˮԡ���ȹ��������ɱ���ʯ�Ļ�ѧ����ʽΪ��2NaF+4NH4F+NaAlO2+2H2O= Na3AlF6��+4NH3��H2O�����ӷ���ʽΪ3Na��+4NH4++6F��+AlO2-+2H2O=Na3AlF6��+4NH3��H2O���ʴ�Ϊ��Na2SiF6+4NH3H2O�T2NaF+4NH4F+ SiO2��+2H2O��3Na��+4NH4++6F��+AlO2-+2H2O=Na3AlF6��+4NH3��H2O��
(4)���������������ڶ��ι���������ҺΪ��ˮ��Һ��Ϊ�����ԭ�������ʣ����ٻ�����Ⱦ����ѭ��ʹ�ð�ˮ���ʴ�Ϊ������2����Һ��ˮ��ѭ�����á�