��Ŀ����
��Na2CO3��NaAlO2�Ļ����Һ����μ���150mL 1mol•L-1��HCl��Һ�������Һ�е�ij�����������ʵ����ı仯��ͼ��ʾ��������˵������ȷ���ǣ� ��
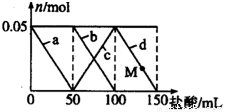
A. a���߱�ʾ�����ӷ���ʽΪ��AlO2-+H++H2O=Al��OH��3
B. b��c���߱�ʾ�����ӷ�Ӧ����ͬ��
C. M��ʱAl��OH��3������С��3.9g
D. ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ2��1
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ



 B.
B.  C.
C.  D.
D. 
 N2O4(g) ��H<0���ﵽƽ�����С�������������˵����ȷ����
N2O4(g) ��H<0���ﵽƽ�����С�������������˵����ȷ����

 H++HCO3��
H++HCO3��