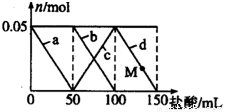
��Ŀ����
A��һ�ֳ�����������ͬ����������������ܶ�Ϊ13��D����С�մ�Ӧ�������壬����֮���������ͼ��ʾ��ת����ϵ����Ӧ������������ȥ����

��ش�
(1)D�к��й����ŵ�������________________��
(2)��Ӧ�ݷ����������Ǽ��Ⱥʹ������仯ѧ����ʽΪ________________��
(3)������ȩ�����������ſ���ѡ�õ��Լ���________________��
(4)����˵����ȷ����________________��
a��������Ӧ�����ڼӳɷ�Ӧ��ֻ�Тٺ͢� b����ȥC�к���D�ɼ���ʯ������
c����ҵ�ϻ�ô���B��ͨ��ʯ���ѻ����� d�������ʵ���B��C��ȫȼ�պ�������ͬ
��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ
��֪H2(g)+Br2(l)=2HBr(g);��H=��72KJ/mol������1molBr2(l)��Ҫ���յ�����Ϊ30KJ������������������±���
H2(g) | Br2(g) | HBr(g) | |
1mol�����л�ѧ������ ʱ��Ҫ���յ�����/kJ | 436 | a | 369 |
�����aΪ
A. 404 B. 260 C. 230 D. 200


 B+HCOOH������ƽ������ϸ÷�Ӧ�������л���B�У����������칹��
B+HCOOH������ƽ������ϸ÷�Ӧ�������л���B�У����������칹��
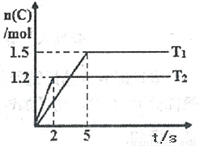
 2C(g)���ֱ���Tl��T2ʱ���������C�����ʵ�����ʱ��仯��ͼ��ʾ������˵����ȷ����
2C(g)���ֱ���Tl��T2ʱ���������C�����ʵ�����ʱ��仯��ͼ��ʾ������˵����ȷ����