��Ŀ����
����Ŀ����+6�۸�����ˮ����Ⱦ��������Ƴ������Ķ�ͭ��ˮ����������һ������![]() �������÷�ˮ���û�ԭ�������������������£�
�������÷�ˮ���û�ԭ�������������������£�

��1���������ϡ�������pH����ѡ�������ԭ����______________________________��
��2����֪ÿ����1mol Na2S2O3ת��8mol e-�������Na2S2O3��Һʱ������Ӧ�����ӷ���ʽΪ��_________________________________________________________��
��3����֪Cr(OH)3�Ļ�ѧ������Al(OH)3���ơ�����ڼ���NaOH��ҺʱҪ������Һ��pH���ܹ��ߣ�����Ϊ___________________________________________(�����ӷ���ʽ��ʾ)��
��4��������Һ�п��Դ�������������Na2S2O3��Һ��ѵ���__________(��ѡ�����)��
A��FeSO4��Һ B��ŨH2SO4C��Na2SO3��Һ D������KMnO4��Һ
��5��ij����ˮ�к�1.50��10-3mol/L��![]() ������ˮ�����õ����Բ���Cr0.5Fe1.5FeO4(Cr�Ļ��ϼ�Ϊ+3��Fe�Ļ��ϼ�����Ϊ+3��+2)�ɱ��Ϊ������ʹ1 L�÷�ˮ�е�
������ˮ�����õ����Բ���Cr0.5Fe1.5FeO4(Cr�Ļ��ϼ�Ϊ+3��Fe�Ļ��ϼ�����Ϊ+3��+2)�ɱ��Ϊ������ʹ1 L�÷�ˮ�е�![]() ��ȫת��ΪCr0.5Fe1.5FeO4����������Ҫ����__________gFeSO4��7H2O��(��֪FeSO4��7H2O��Ħ������Ϊ278g/mol)
��ȫת��ΪCr0.5Fe1.5FeO4����������Ҫ����__________gFeSO4��7H2O��(��֪FeSO4��7H2O��Ħ������Ϊ278g/mol)
���𰸡�![]() ������������C1-������������Ⱦ����
������������C1-������������Ⱦ���� ![]() Cr(OH)3+OH-=
Cr(OH)3+OH-=![]() +2H2O C 4.17
+2H2O C 4.17
��������
��Cr2O72-���ӵķ�ˮ����Na2S2O3��Һ������ҺpH=2~3���ظ�������ӱ���ԭΪCr3+���ӣ��ټ�������������Һ������ҺpH����Cr��OH��3��Cr(OH)3�Ļ�ѧ������Al(OH)3���ƣ������������м���NaOH��ҺʱҪ������Һ��pH���ܹ��ߣ���ֹCr(OH)3�ܽ⡣
��1��������������Cr2O72-����ǿ�����ԣ�����Cl- ���ӷ���������ԭ��Ӧ��������������ɻ�����Ⱦ���������������������
��2����֪ÿ����1mol Na2S2O3ת��8mol e-�����ݵ���ת���غ㣬��֪S2O32����������SO42����Cr2O72���ӱ���ԭΪCr3+���ӣ���ƽ�ɵ����ӷ���ʽΪ��3S2O32+4Cr2O72+26H+�T6SO42+8Cr3++13H2O��
��3��Cr(OH)3�Ļ�ѧ������Al(OH)3���ƣ�Cr(OH)3�����ܽ��ڹ���������������Һ�У���Ӧ����NaCrO2��H2O����Ӧ���ӷ���ʽΪ��Cr(OH)3+OH=CrO2+2H2O��
��4�����Դ�������������Na2S2O3��Һ����Ҫ���л�ԭ�ԣ��ܻ�ԭ�ظ�������ӣ�
A��FeSO4��Һ ���������Ӿ��л�ԭ�ԣ����Ի�ԭCr2O72���ӣ������������µ��������������ӣ���A�����ϣ�
B��ŨH2SO4 ���������ԣ���B�����ϣ�
C��Na2SO3��Һ ������������Ӿ��л�ԭ��,���Ի�ԭCr2O72����C���ϣ�
D������KMnO4��Һ��ǿ���������ܻ�ԭCr2O72����D�����ϣ�
�ʴ�ѡC��
��5��1L��ˮ�к�n(Cr2O72)=1.50��103mol������Crԭ�ӡ�Feԭ���غ㣬�ɵã�
Cr2O724Cr0.5Fe1.5FeO410FeSO47H2O��
����������n(FeSO47H2O)=10n(Cr2O72)=1.50��103mol��10=0.015mol��
����m(FeSO47H2O)��0.015mol��278g/mol=4.17g��

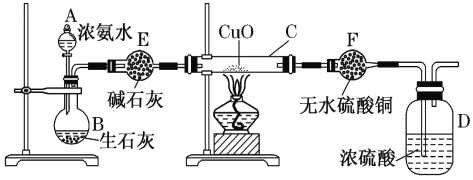
����Ŀ������ͼװ�ý���ʵ�飬�����ƶ���ȷ����(����)
ѡ�� | �����Լ� | �����Լ������� | �ƶ� |
A | �Ȼ�� | ��̪��Һ�����ɫ | �Ȼ���ȶ� |
B | �������� | Ʒ����Һ��ɫ | FeSO4�ֽ�����FeO��SO2 |
C | Ϳ��ʯ���͵����Ƭ | ���Ը��������Һ��ɫ | ʯ���ͷ����˻�ѧ�仯 |
D | ������ˮ���� | ����ˮð�� | ������ˮ���������˷�Ӧ |
A. A B. B C. C D. D