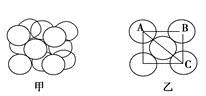��Ŀ����
������BN����һ����Ҫ�Ĺ����մɲ��ϡ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN����ش���������
��1����̬Bԭ�ӵĵ����Ų�ʽΪ_________��B��N��ȣ��縺�Խϴ����_________��BN��BԪ�صĻ��ϼ�Ϊ_________��
��2����BF3�����У�F-B-F�ļ�����_______��Bԭ�ӵ��ӻ��������Ϊ_______��BF3����NaF���ÿ�����NaBF4��BF4��������ṹΪ_______��
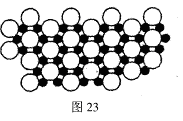
��3������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ________�����������Ϊ________��
��4�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�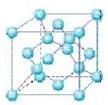 Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к���______����ԭ�ӡ�________����ԭ�ӣ�������������ܶ���_______g��cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к���______����ԭ�ӡ�________����ԭ�ӣ�������������ܶ���_______g��cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
��(1)1s22s22p1�� N�� +3 (2)120�� �� sp2�� ��������
(3)���ۼ�(���Թ��ۼ�)�� ���Ӽ���
(4)4�� 4�� 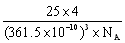
���������������1��5��Ԫ��B�Ļ�̬Bԭ�ӵĵ����Ų�ʽΪ1s22s22p1��B��N��ȣ�����ԭ�Ӱ뾶B>N���ǽ�����N>B�����Ե縺�Խϴ����N����BN��BԪ�صĻ��ϼ�Ϊ+3�ۡ���2��BF3������ƽ���������η��ӡ���BF3�����У�F-B-F�ļ�����120�㡣Bԭ�ӵ��ӻ��������Ϊsp2��BF3����NaF���ÿ�����NaBF4����BF4����Bԭ�ӵ��ӻ���ʽΪsp3����������ṹΪ�������塣��3������������ʯī�ṹ���ơ��ڲ���Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ���Թ��ۼ����ڲ���������Ƿ��Ӽ�����������4������������Ľṹ����ʯ���ƣ�Ӳ������ʯ�൱���ڽ��ʯ��һ�������к���C��8��1/8��6��1/2+4=8.���������������к���4����ԭ�ӡ�4��ԭ�ӣ����ھ����߳�Ϊ361.5pm������������������ܶ���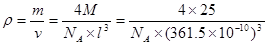 g��cm-3��
g��cm-3��
���㣺����ԭ�ӡ����Ӽ�����Ľṹ�������ܶȵļ����֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���14�֣�Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���������������Ϣ���ɣ������ǰ��ֶ�����Ԫ�ص������Ϣ����֪���ԭ�Ӱ뾶Ϊ0.089nm��
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -2 |
��1��BԪ����Ԫ�����ڱ��е�λ��___________________����������Ԫ�ص�����������Ӧ��ˮ������������ǿ����__________��A���ӵĽṹʾ��ͼ_______________��
��2���õ���ʽ��ʾA��D�γɻ�����Ĺ��̣�____________________________________��H��E�γ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽΪ___________�����еĻ�ѧ������Ϊ____________��C2D2�ĵ���ʽΪ______________________��
��3������˵����˵��D�ķǽ����Ա�Cǿ��ѡ��____________
��H2CO4��HDO�ȶ���HDO4��H2CO4����ǿ��C2-��D-�ױ�������HD��H2C�ȶ���ͭ��HD����Ӧ��������ŨH2CO4��Ӧ������D2��������FeD3������C��������FeC��Cԭ����Dԭ�ӵ��Ӳ�����ͬ��Dԭ�Ӱ뾶С��Cԭ�ӡ�
A��ȫ�� B���ڢۢܢޢ� C���٢ڢܢݢ� D����������
��4��A��B��C��D��E�γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________________�����þ������ӷ��ű�ʾ��
��5��C������H������������Ӧˮ�����ڼ����������ܷ�����Ӧ������3mol��C���뷴Ӧ��ת��4NA�ĵ��ӣ���д�����ӷ�Ӧ����_______________________________________���������뻹ԭ��������֮��_____________________��
���и��黥Ϊ�ȵ��������
| A��N2O��NO2 | B��O3��SO2 | C��CH4��NH3 | D��OH-��NH2- |