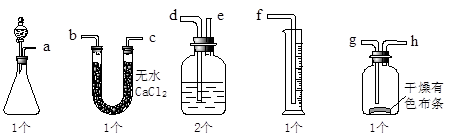��Ŀ����
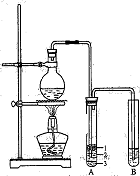
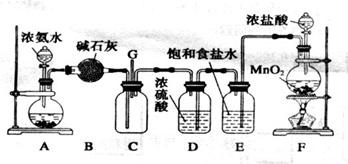
ijͬѧӦ��������ʾװ���о����ʵ����ʡ���������C����Ҫ�ɷ�������������������������ˮ��������ش��������⣺

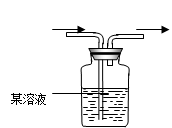
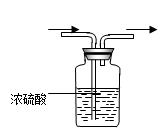
(1�������о�����ҪĿ����̽��������ˮ��Ӧ�����ɵ����ʾ��� ����
��2��Ũ����������� ��
��3���۲쵽��ʵ��������B�� ��C�� ��
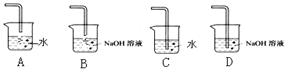
��4�����������ʷ���������������ʵ����ƻ������¹��������¹ʱ����� ��Ҫ�˷����¹�������ѡ��IJ�������ͼ�� ,
��д������������ص����ӷ���ʽ��ʾ��1�֣��� ��

(1�������о�����ҪĿ����̽��������ˮ��Ӧ�����ɵ����ʾ��� ����
��2��Ũ����������� ��
��3���۲쵽��ʵ��������B�� ��C�� ��
��4�����������ʷ���������������ʵ����ƻ������¹��������¹ʱ����� ��Ҫ�˷����¹�������ѡ��IJ�������ͼ�� ,

��д������������ص����ӷ���ʽ��ʾ��1�֣��� ��
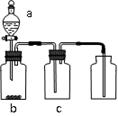
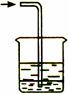
��7�֣�(1��Ư���� ��2����������A�е�ˮ
��3��B�и���IJ�������ɫ�� C��ʪ��IJ�����ɫ
��4��û��β������װ�ã���ɻ�����Ⱦ D Cl2 + 2OH- = Cl- + ClO- + H2O
��3��B�и���IJ�������ɫ�� C��ʪ��IJ�����ɫ
��4��û��β������װ�ã���ɻ�����Ⱦ D Cl2 + 2OH- = Cl- + ClO- + H2O
�����������1������װ�����и������ɫ������֪�������о�����ҪĿ����̽��������ˮ��Ӧ�����ɵ����ʾ���Ư���Եġ�
��2�����������к���ˮ����������Ũ��������þ�����������A�е�ˮ����ֹ����ʵ�顣
��3������������Ư�����õ���������ˮ��Ӧ���ɵĴ����ᣬ����ʵ���������B�и���IJ�������ɫ����C��ʪ��IJ�����ɫ��
��4�����������ж��������ŷŻ���ɻ�����Ⱦ�����Ը�ʵ��װ�û�ȱ��β������װ�á���������һ��������������Һ����Ȼװ��D���������йط�Ӧ�����ӷ���ʽ��Cl2 + 2OH- = Cl- + ClO- + H2O��
�������������е��Ѷȵ����⣬����ע�ػ��������������������ͽ��ⷽ����ѵ��������������ѧ������˼ά���������ѧ���Ĺ淶ʵ����ƣ�Ҳ���������ѧ���������⡢�Լ�������û���֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ