��Ŀ����
(1)�����뺬�Ȼ������йص�˵����ȷ����________(����ĸ)��
| A��HClO�����ᣬ����NaClO��������� |
| B�����ˮ����μ�����������FeCl3��Һ�����Ƶ�Fe(OH)3���� |
| C��HCl��Һ��NaCl��Һ��ͨ�����ӵ��磬����HCl��NaCl�������ӻ����� |
| D�����NaCl��Һ�õ�22.4 L H2(��״��)����������Ҫת��NA������(NA��ʾ�����ӵ�����) |
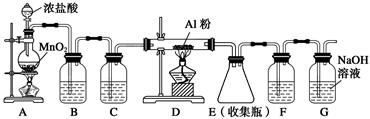
װ��B��ʢ�ű���NaCl��Һ����װ�õ���Ҫ������______________��F���Լ���������____________________����һ������װ���ʵ��Լ����ͬʱ��F��G�����ã���װ����Լ�Ϊ________________��
(1)B��(2)��ȥ������HCl�������������ܽ�ȡ���ֹˮ��������E����ʯ��
����

��ϰ��ϵ�д�
�����Ŀ
�о�CO��SO2��NO�ȴ�����Ⱦ������ۺϴ��������þ�����Ҫ���塣
��1����CO��CO2��H2Ϊԭ�ϣ���һ�������¾��ɺϳɼ״�������Ϊ�����ֺϳ������·�����ϡ���ɫ��ѧ��������û�ѧ��Ӧ����ʽ��ʾ�� ��
��2����ͼ��ʾ�����ںϳɼ״���Ʒ�м״������ļ���ǡ�д������������ʱ�ĵ缫��Ӧʽ��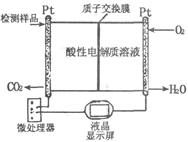
���� ������ ��
��3��һ�������£�NO2��SO2��Ӧ����SO3��g����NO�������壬�ֽ������Ϊ1:2��NO2��SO2�Ļ�����������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��������ţ�
| A����ϵѹǿ���ֲ��� | B�����������ɫ���ֲ��� |
| C��SO3��NO������ȱ��ֲ��� | D��ÿ���� 1 mol SO2��ͬʱ����1 mol NO |
��4����ҵ����Na2CO3������Һ����NO��NO2���壺
NO+NO2+Na2CO3=2NaNO2+CO2 2NO2+Na2CO3=NaNO3+NaNO2+CO2
����������Na2CO3��Һ��ȫ����NO��NO2������壬ÿ������״����CO2 2��24L��CO2����ȫ���ݳ���ʱ������Һ����������4��4g������������NO��NO2�����Ϊ ��
���Ʋ�����ԭ���У��ټ����������ʺ��Ƶø��ֲ�ͬ��;�IJ�����
| A��������ͭ(Cu2O) | B��������(Co2O3) |
| C������Ǧ(PbO) | D����ɰ(Na2B4O7��10H2O) |
(2)�����ѧ�����IJ���������������______��
(3)������ɫ����������������____________��
(4)�����ɫ����������������____________��
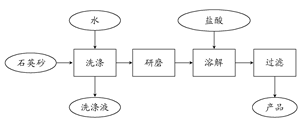
 Si��SiC��4CO��
Si��SiC��4CO�� SiHCl3��H2��
SiHCl3��H2��


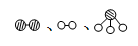 �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� �� 7N2��12H2O��NOҲ�����Ƶķ�Ӧ��
7N2��12H2O��NOҲ�����Ƶķ�Ӧ�� Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)