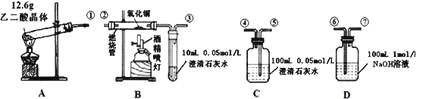
��Ŀ����
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
����
����ʽ��HCl
��Է���������36.5
�ܶȣ�1.19 g��cm-3
HCl������������36.5%
��1����Ũ������HCl�����ʵ���Ũ��Ϊ_________mol��L ��1 ��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����________��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl�� ����Ŀ D����Һ���ܶ�
��3��ijѧ��ȡ����Ũ����100mL��
������ˮϡ�͵�500 mL��������������ʵ���Ũ��Ϊ________mol��L ��1 ��
������ʹ����Һ������������Ϊԭ����![]() ��Ӧ�ü�______mL����ˮ��
��Ӧ�ü�______mL����ˮ��
������ʹ����Һ�����ʵ���Ũ�ȱ�Ϊԭ����![]() ���������ˮ�����______119mL���ÿ��>����<����=������
���������ˮ�����______119mL���ÿ��>����<����=������
���𰸡� 11.9 )BD 2.38 119 <
����������1��1L��Һ����������ʵ����ǣ�1.19��1000��36.5%/36.5 mol��11.9mol���ʸ������Ũ����11.9mol/L����2��A����Һ��HCl�����ʵ�������Һ����йأ�A��ѡ��B����Һ��Ũ������Һ������أ�Bѡ��C����Һ��Cl-����Ŀ����Һ������йأ�C��ѡ��D����Һ���ܶ�����Һ������أ�Dѡ����ѡBD����3��������ˮϡ�͵�500 mL����ϡ��5������������������ʵ���Ũ��Ϊ11.9mol/L��5��2.38mol/L��������ʹ����Һ������������Ϊԭ����1/2������Һ����Ӧ����ԭ����2����ԭ��Һ������1.19g/mL��100mL��119g������Ӧ�ü�119mL����ˮ��������ʹ����Һ�����ʵ���Ũ�ȱ�Ϊԭ����1/2������Һ�����Ӧ����ԭ����2�������������Һ�ܶȲ��䣬���������ˮ�����Ϊ119mL��ˮ���ܶ�С��������ܶȣ�ϡ�ͺ���Һ���ܶȼ�С�������Ҫˮ�����ӦС��119mL��

 ����������������ϵ�д�
����������������ϵ�д�����Ŀ��ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��ж������ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ���������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
���� | Al | Al2O3 | Fe | Fe2O3 |
�۵�/�� | 660 | 2054 | 1535 | 1462 |
�е�/�� | 2467 | 2980 | 2750 | -- |
������1��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ�����Ϊ���Ľ����Ƿ��������_____ ��������������������������
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���_____ ����Ӧ�����ӷ���ʽΪ____ __��
��3��ʵ�����ܽ������������Լ�����õ���___ ___������ţ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ
����ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4molL-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
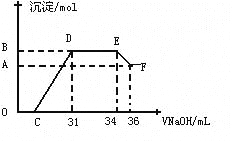
��4��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ____ __
��5����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ____ _ ����������˵����Һ��_____ _���OH����������___ ___ǿ�������ӷ��ţ���
��6��B���Ӧ�ij��������ʵ���Ϊ____ __mol��C���Ӧ������������Һ�����Ϊ____ __mL��
����Ŀ��������֧�Թ��У���������ֽ���������Ļ�ѧ��Ӧ���������ǣ�������
�Թ� | �¶� | ��������Ũ�� | ���� |
A | ���£�25�棩 | 12% | �� |
B | ˮԡ���ȣ�50�棩 | 4% | �� |
C | ˮԡ���ȣ�50�棩 | 12% | �� |
D | ���£� 25�棩 | 4% | �� |
A.A
B.B
C.C
D.D