��Ŀ����
����Ŀ����ѧ�ҷ��ֶ�ұ�����е�⾫���ᴿ�ɽ��ߴ����Ʊ��ɱ�����ص���װ����ͼ��ʾ����Cu-Si�Ͻ�����Դ����950����������Һ���ν��е�⾫�����й�˵������ȷ����
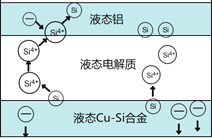
A. �ڸ�Һ��������Cu������Si��������Si4+������Cu2+����ԭ
B. ������Һ̬Cu-Si�Ͻ���������Һ̬������
C. ����Һ���ε������������ⷴӦ�������߹����Ч��
D. ����ǿ�Ȳ�ͬ����Ӱ����ᴿ����
���𰸡�A
����������ͼʾ�õ�������������ӦΪSiʧ����ת��ΪSi4+��������ӦΪSi4+�õ���ת��ΪSi������ѡ��A������ͼʾ�õ���Һ̬��Ϊ���������ӵ�Դ���������Ե��Ӵ�Һ̬�����룻Һ̬Cu-Si�Ͻ�Ϊ������������Һ̬Cu-Si�Ͻ�������ѡ��B��ȷ��ʹ������Һ���εĿ�����Ч�������ⷴӦ�������ʹ���ʹ��Ч����Һ̬���缫�ϳ�����ѡ��C��ȷ����ⷴӦ������һ���ɵ���ǿ�Ⱦ���������ѡ��D��ȷ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��Ϊ�ᴿ��������(�����ڵ�����Ϊ����)����ѡ�õij����Լ�����뷽������ȷ����
ѡ�� | �ᴿ������ | �����Լ� | ���뷽�� |
A | ��Ȳ(����) | ����ͭ��Һ | ϴ�� |
B | ��(����) | Ũ��ˮ | ���� |
C | �Ҵ�(����) | ��ʯ�� | ���� |
D | ������(����������) | ˮ | �ؽᾧ |
A. A B. B C. C D. D