��Ŀ����
��8�֣�����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2(g)��O2(g)===2H2O(l)����H����572 kJ��mol��1����ش��������⣺
(1)�����������ܺ�________(����ڡ�����С�ڡ����ڡ�)��Ӧ�������ܺ͡�
(2)��2 mol������ȫȼ������ˮ��������ų�������________(����ڡ�����С�ڡ����ڡ�)572 kJ��
��3����Ӧ2H2+ O2 �� 2H2O �������仯��ͼ��ʾ����֪��1molH2��1molO2��1molH-O�еĻ�ѧ���ֱ���Ҫ����436KJ��496KJ��463KJ��������Ӧ���̣��� ������ա��ų����� KJ��
(1)С�ڡ�(2)С�ڡ���3���ų� 1852
����:���黯ѧ��Ӧ�е������仯
��1����Ӧ�Ƿ��ȷ�Ӧ�����Է�Ӧ����������������������������
��2����̬ˮ����������Һ̬ˮ����������������ȼ��������̬ˮ�ų��������͡�
��3������ͼ���֪����Ӧ���̣������γɻ�ѧ���ģ��Ƿ��ȹ��̣��ų���������2��2��463kJ��1852kJ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
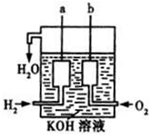 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��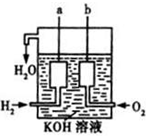 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��