��Ŀ����
��17�֣�������Ⱦ������������ȫ���������ŵĿ��⡣
��1��Ϊ�˼��ٿ�����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
�ٽ�úת��Ϊ�������ȼ�ϡ�
��֪��H2(g)��1/2O2(g)==H2O(g) ��H1��-241.8 kJ��mol��1
C(s)��1/2O2(g)===CO(g) ��H2����110.5 kJ��mol��1
��̿��ˮ������Ӧ����CO���Ȼ�ѧ����ʽΪ ��
��ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ����� ������ţ���
a��Ca(OH)2 b��CaCl2 c��Na2CO3 d��NaHSO3
��2��CO�ڴ��������¿�����H2��Ӧ���ɼ״���CO(g)+2H2(g) CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��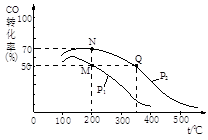
��M��N����ƽ��״̬�£������������ʵ����ʵ���֮��Ϊ��n(M)����n(N)�������� ��������
����M��N��Q�����ƽ�ⳣ��KM��KN��KQ�Ĵ�С��ϵΪ���� ��
��3�����������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
�ٴ��������У���H2��NO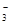 ��ԭΪN2��һ��ʱ�����Һ�ļ���������ǿ����÷�Ӧ���ӷ���ʽΪ ��
��ԭΪN2��һ��ʱ�����Һ�ļ���������ǿ����÷�Ӧ���ӷ���ʽΪ ��
�ڵ绯ѧ����NO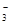 ��ԭ������ͼ��ʾ����Դ����Ϊ ���a����b���������ܷ�ӦΪ4NO3-+4H+ͨ��5O2��+2N2��+2H2O����������ӦʽΪ ��
��ԭ������ͼ��ʾ����Դ����Ϊ ���a����b���������ܷ�ӦΪ4NO3-+4H+ͨ��5O2��+2N2��+2H2O����������ӦʽΪ ��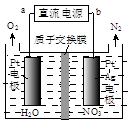
��1����4�֣��� C(s)+H2O(g)��CO(g) +H2(g) ��H��+131.3kJ��mol��1��2�֣� �� a c��2�֣�
��2����5�֣��� 5��4��2�֣� ��KM��KN��KQ��3�֡�˵���������߿��¶�����COת���ʽ��ͣ��÷�Ӧ������Ӧ���ȣ�M��N�����¶���ͬK��ͬ��C���¶�����ƽ�����淽���ƶ���COת���ʽ��ͣ���Kֵ��С�� ��3����8�֣���2NO3��+5H2����N2+2OH��+4H2O��3�֣�
��a��2�֣� 2NO3��+12H++10e����N2��+6H2O
��3�֡�������ӦΪ��4OH-��4e-��O2��+2H2O��10H2O-20e-��20H++5O2����
���������������1������֪��Ӧ����H2(g)��1/2O2(g)==H2O(g) ��H1��-241.8 kJ��mol��1����C(s)��1/2O2(g)��CO(g) ��H2����110.5 kJ��mol��1������ݸ�˹���ɿ�֪�ڣ��ټ��õ���̿��ˮ������Ӧ����CO���Ȼ�ѧ����ʽ����C(s)+H2O(g)��CO(g) +H2(g) ��H��+131.3kJ��mol��1��
���������ƺ�̼���ƾ���SO2��Ӧ��������Ϊϴ�Ӽ����Ȼ�����������������SO2������Ӧ��������Ϊϴ�Ӽ�����ѡac��
��2����M��CO��ת����Ϊ0.5����μӷ�Ӧ��COΪ10mol��0.5=5mol����
CO��g��+2H2��g�� CH3OH��g���������ʵ�������
CH3OH��g���������ʵ�������
2
5mol 10mol
��M��ƽ��ʱ����������ܵ����ʵ���=10mol+20mol-10mol=20mol
N��CO��ת����Ϊ0.7����μӷ�Ӧ��COΪ10mol��0.7=7mol����
CO��g��+2H2��g�� CH3OH��g���������ʵ�������
CH3OH��g���������ʵ�������
1 2
7mol 14mol
��N��ƽ��ʱ����������ܵ����ʵ���=10mol+20mol-14mol=16mol
��M��N����ʱ����������������ʵ���֮��n��M����n��N��=20mol��16mol=5��4��
����ͼ��֪��һ��ѹǿ�£��¶�Խ�ߣ�CO��ת����Խ�ͣ�˵�������¶�ƽ�����淴Ӧ�����ƶ���Q����¶ȸ���M��N�㣬��ƽ�ⳣ��KM��KN��KQ��
��3������H2��NO ��ԭΪN2��һ��ʱ�����Һ�ļ���������ǿ����˵����Ӧ�������������ɣ���÷�Ӧ���ӷ���ʽΪ2NO3��+5H2����N2+2OH��+4H2O��
��ԭΪN2��һ��ʱ�����Һ�ļ���������ǿ����˵����Ӧ�������������ɣ���÷�Ӧ���ӷ���ʽΪ2NO3��+5H2����N2+2OH��+4H2O��
����Һ���������������ŵ����������������װ��ͼ��֪���������ĵ缫���������������������Դ����������������a�ǵ�Դ�������������ܷ�ӦΪ4NO3-+4H+ͨ��5O2��+2N2��+2H2O��֪������������õ��������ɵ�������������ӦʽΪ2NO3��+12H++10e����N2��+6H2O��
���㣺�����Ȼ�ѧ����ʽ����д��SO2�����ʡ����淴Ӧ���йؼ������ж��Լ��绯ѧԭ����Ӧ��

����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
| ������ | ��̿���ڸ��������»�ԭCuO |
| ������ | ��ⷨ����ӦΪ2Cu + H2O  Cu2O + H2���� Cu2O + H2���� |
| ������ | ���£�N2H4����ԭ����Cu(OH)2 |
��1����ҵ�ϳ��÷�����ͷ�������ȡCu2O�������÷�������ԭ���Ƿ�Ӧ���������ƣ������²��������� ��ʹCu2O���ʽ��͡�
��2����֪��2Cu(s)��1/2O2(g)=Cu2O(s) ��H =-akJ��mol-1
C(s)��1/2O2(g)=CO(g) ��H =-bkJ��mol-1
Cu(s)��1/2O2(g)=CuO(s) ��H =-ckJ��mol-1
�������ķ�Ӧ��2CuO(s)��C(s)= Cu2O(s)��CO(g)����H = kJ��mol-1��
��3��������������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ���õ�ص���������Cu2O��ӦʽΪ ��

��4��������Ϊ������������Һ̬�£�N2H4����ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2�����Ʒ��Ļ�ѧ����ʽΪ ��
��5������ͬ���ܱ������У����������ַ����Ƶõ�Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺

ˮ������Ũ�ȣ�mol/L����ʱ��t(min)�仯���±���ʾ��
| ��� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
A��ʵ����¶�T2С��T1
B��ʵ���ǰ20 min��ƽ����Ӧ����v(O2)=7��10-5 mol��L-1 min-1
C��ʵ��ڱ�ʵ������õĴ�����Ч�ʸ�
�������е��Լ���ȥ�����е����ʣ�����ȷ����
| A�����еļױ�(��ˮ) | B���Ҵ��е�ˮ(����CaO) |
| C�������е���ϩ(��ˮ) | D�����������е�����(����Na2CO3��Һ) |


 3Zn+2K2FeO4+8H2O,�õ�طŵ�ʱ�������ϵĻ�ѧʽ�� ��缫��Ӧʽ��______������·��ͨ��1.204��1023������ʱ�����������仯Ϊ______g���ʱ,�õ�ص�������ֱ����Դ��______(�����������������
3Zn+2K2FeO4+8H2O,�õ�طŵ�ʱ�������ϵĻ�ѧʽ�� ��缫��Ӧʽ��______������·��ͨ��1.204��1023������ʱ�����������仯Ϊ______g���ʱ,�õ�ص�������ֱ����Դ��______(�����������������