��Ŀ����
����Ŀ��300��ʱ��������X������Y��1.6 mol����10 L�����ܱ������У�������Ӧ��X(g) +Y(g)2Z(g) ��H��0��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������±���
t/min | 2 | 4 | 7 | 9 |
n(Y)/mol | 1.2 | 1.1 | 1.0 | 1.0 |
�ش��������⣺
��1����Ӧ0~2 min Z��ƽ������v(Z)=________________
��2���¶�Ϊ300��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��____________________
��3����ͼ��ʾ�÷�Ӧ�仯���������ʵ�Ũ���뷴Ӧ��ʱ��仯��ϵ��ͼ��t2��t3������߱仯�������������������ĸı��������_______
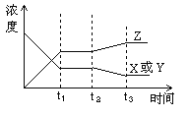
A��������ѹǿ B�������¶�
C��������x��y��Ũ�� D��ʹ���˴���
��4������ʼʱ��������г���X��Y��Z��2.0 mol����Ӧ����_______����������������������Ӧ������У���ƽ��ʱZ���������=____________��ƽ��ʱn(Y)=_______________��
���𰸡�0.04 mol/L/ min 1.44 B �� 0.375 1.875 mol
��������
��1���ɱ������ݺͻ�ѧ��Ӧ���ʹ�ʽ���㣻
��2����������ʽ���㣻
��3������ͼ���ж�ƽ���ƶ��������ݻ�ѧƽ���ƶ�ԭ��������
��4������Ũ���غ�ƽ�ⳣ���Ĵ�С�ж�ƽ���ƶ�����������ʽ���㡣
��1���ɱ������ݿ�֪��0~2 min��Y��Ũ�ȱ仯��Ϊ(0.16-0.12)mol/L=0.04mol/L���ɻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪��v(Z)=2v(Y)=2��![]() =0.04mol/(L��min)���ʴ�Ϊ��0.04mol/(L��min)��
=0.04mol/(L��min)���ʴ�Ϊ��0.04mol/(L��min)��
��2���ɱ������ݿ�֪��7minʱ��Ӧ�ﵽƽ�⣬�����⽨����������ʽ��
X(g) + Y(g) ![]() 2Z(g)
2Z(g)
��mol/L��0.16 0.16 0
�䣨mol/L��0.06 0.06 0.12
ƽ��mol/L��0.1 0.1 0.12
��Ӧ�Ļ�ѧƽ�ⳣ��K��![]() =
=![]() =1.44���ʴ�Ϊ��1.44��
=1.44���ʴ�Ϊ��1.44��
��3����ͼ��֪��t2��t3��������Z��Ũ������Ӧ��X��Y��Ũ�ȼ�С��ƽ��������Ӧ�����ƶ���
A���÷�Ӧ�������������ķ�Ӧ������ѹǿ��ƽ�ⲻ�ƶ����ʴ���
B���÷�Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ���������Z��Ũ������Ӧ��X��Y��Ũ�ȼ�С������ȷ��
C��������X��Y��Ũ�ȣ������ı��t2ʱ�̣�X��Y��Ũ��Ӧ���ʴ���
D��ʹ�ô�����ƽ�ⲻ�ƶ����ʴ���
B��ȷ���ʴ�Ϊ��B��
��4������ʼʱ��������г���X��Y��Z��2.0mol��Ũ����Qc��![]() =
=![]() =1��K��ƽ��������Ӧ�����ƶ�����A��������Ϊa�������⽨����������ʽ��
=1��K��ƽ��������Ӧ�����ƶ�����A��������Ϊa�������⽨����������ʽ��
X(g) + Y(g) ![]() 2Z(g)
2Z(g)
��mol/L��0.2 0.2 0.2
�䣨mol/L��a a 2a
ƽ��mol/L��0.2-a 0.2-a 0.2+2a
��Ӧ�Ļ�ѧƽ�ⳣ��K��![]() =
=![]() =1.44�����a=0.0125mol/L����Z���������Ϊ
=1.44�����a=0.0125mol/L����Z���������Ϊ![]() =37.5%��ƽ��ʱn(Y)=(0.2-0.0125)mol/L��10L=1.875mol���ʴ�Ϊ��0.375��1.875��
=37.5%��ƽ��ʱn(Y)=(0.2-0.0125)mol/L��10L=1.875mol���ʴ�Ϊ��0.375��1.875��

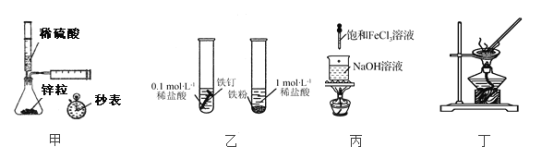
����Ŀ������98%��Ũ����(g��1.84g��cm��3)���Ƴ�Ũ��Ϊ0.5mol��L��1��ϡ����500ml��
(1)ѡ�õ���Ҫ�����У�
��__________����__________����__________����____________����____________��
(2)�뽫���и�����������ȷ��������ں����ϡ�
A������Ͳ��ȡŨH2SO4 |
B�������ߵ�ҡ�� |
C���ý�ͷ�ιܼ�����ˮ���̶��� |
D��ϴ���������� |
E��ϡ��ŨH2SO4
F������Һת������ƿ
�������ȷ��˳������Ϊ______________________��
(3)��Ҫ�ش��������⣺
������Ũ��������Ϊ____________mL��
�����ʵ������15mL��20mL��50mL����ͲӦѡ��____________mL����Ͳ��ã���ȡʱ������Ͳ���ɾ���ˮϴ����ֱ����ȡ��ʹŨ��__________(��ƫ��������ƫ����������Ӱ����)
����Ũ�������ձ��ڱ�����ע��ʢˮ���ձ��У����ò��������Ͻ����Ŀ����____________���������������Һ�彦�������ʹŨ��ƫ____________��
����ת������ƿǰ�ձ���Һ��Ӧ____________�������ʹŨ��ƫ____________����ϴ���ձ��Ͳ�����2��3�Σ�ϴ��ҺҲҪת������ƿ�������ʹŨ��____________��
������ʱ����ʹ��Һ��Һ����̶������У������ӻ�ʹŨ��____________��������ʹŨ��___________��