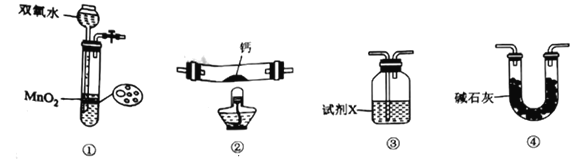
��Ŀ����
����Ŀ����֪ͭ��Ũ��������ڼ��������·������·�Ӧ:Cu��2H2SO4(Ũ)![]() CuSO4��A����2H2O����ͨ�����������������������:
CuSO4��A����2H2O����ͨ�����������������������:
(1)A���ʿ���ʹ����KMnO4��Һ___________(ʵ������)����Ӧ�еĻ�ԭ����____________����ѧʽ����
(2)һ������ͭƬ��100mL18mol/L ��ŨH2SO4��ַ�Ӧ������÷�Ӧ������ת����0.2mol���ӣ����ɵ�CuSO4������Ϊ_________g, ���ɵ�A�����ڱ�״���µ����Ϊ_________L(��������ȫ���ݳ�)��
(3)����Ӧ�����õ�����Һ������Ba(OH)2��Һ��ַ�Ӧ�����ó���Ϊ�����������ᱵ������Ϊ______g��(������0.1g����Ҫ��������)
���𰸡���ɫ SO2 16 2.24 396.1
��������
��1����ԭ�Ӹ����غ㶨�ɿ�֪��Ӧ2H2SO4(Ũ)+Cu![]() CuSO4+SO2��+2H2O�У�AΪSO2������������л�ԭ�ԣ��ܹ������Եĸ����������������������ӣ�
CuSO4+SO2��+2H2O�У�AΪSO2������������л�ԭ�ԣ��ܹ������Եĸ����������������������ӣ�
��2������2H2SO4(Ũ)+Cu![]() CuSO4+SO2��+2H2O��֪ת��2mol��������1mol����ͭ��1mol�����������壻
CuSO4+SO2��+2H2O��֪ת��2mol��������1mol����ͭ��1mol�����������壻
��3����Ӧ��ʣ����ҺΪ���������ͭ��Һ��������������������������������Ӧ�������ᱵ��ˮ������ͭ������������Ӧ�������ᱵ��������ͭ������
(1)ͭ��Ũ��������ڼ��������·������·�Ӧ(��Ӧ����ʽ����ƽ)�� 2H2SO4(Ũ)+Cu![]() CuSO4+SO2��+2H2O������ԭ�Ӹ����غ��֪AΪ����������������л�ԭ�ԣ�����ʹ����KMnO4��Һ��ɫ���仹ԭ����SO2���ʴ�Ϊ����ɫ��SO2��
CuSO4+SO2��+2H2O������ԭ�Ӹ����غ��֪AΪ����������������л�ԭ�ԣ�����ʹ����KMnO4��Һ��ɫ���仹ԭ����SO2���ʴ�Ϊ����ɫ��SO2��
(2)����ת�Ƶ����غ���ܽ�n(Cu)=0.2mol/2=0.1mol������Cuԭ���غ��n(CuSO4)=n(Cu)=0.1mol������ͭ����=nM=0.1mol��160g/mol=16g������ת�Ƶ����غ�ö��������������=0.2mol/2��22.4L/mol=2.24 L���ʴ�Ϊ��16��2.24��
(3)��Ӧ��ʣ����ҺΪ���������ͭ��Һ�����ݵڣ�2���ʿ�֪��n(CuSO4)=n(Cu)=0.1mol����������������Ӧ�����ɵ�������ͭ����������ͭ��Ӧ���ĵ���������ʵ���Ϊ2n(Cu)=0.2mol����Ӧ���ʣ�����������ʵ���n(H2SO4)=18mol/L��0.1L-0.2mol=1.6mol������Һ�е��ܵ���������ӵ����ʵ���Ϊn(CuSO4)+ n(H2SO4)=1.6mol+0.1mol=1.7mol�����������������������������ᱵ�����ʵ���n(Ba SO4)=1.7mol��������Ϊ1.7mol��233g/mol=396.1g��

����Ŀ����֪Ca(OH)2�������(CaWO4)�����ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������ұ�������У����������Ƽ��������Ƽ�����Һ�еõ�����ƣ�������Ӧ��WO42��(aq)�� Ca(OH)2(s) ![]() CaWO4(s)��2OH��(aq)��
CaWO4(s)��2OH��(aq)��
(1)��ͼΪ��ͬ�¶���Ca(OH)2��CaWO4�ij����ܽ�ƽ�����ߡ�

�ټ���T1ʱKsp(CaWO4)��________��
��T1________T2(�>������������<��)��
(2)��Ӧ���ƽ�ⳣ��K����ֵ�����ʾ��
�¶�/�� | 25 | 50 | 90 | 100 |
K | 79.96 | 208.06 | 222.88 | 258.05 |
�ٸ÷�Ӧƽ�ⳣ��K�ı���ʽΪ________________��
�ڸ÷�Ӧ�Ħ�H________0(�>������������<��)��
��������Һ�����Ӽ������ã�ʵ���õ�ƽ�ⳣ��������ֵ�����Զ��50��ʱ����һ������������Ƽ�����Һ[c(Na2WO4)��c(NaOH)��0.5mol��L��1]�У��������Ca(OH)2����Ӧ�ﵽƽ���WO42���ij�����Ϊ60%������ʵ���õ�ƽ�ⳣ��________________��
(3)��ȡ�����ʱ����ʱ��Ӧ���Һ�������������ᣬ�������ã� __________________��