��Ŀ����
����Ŀ����ͼ�ش��������⣺
��.(1)���ձ�����ҺΪϡ���ᣬ��۲쵽��������__________��������ӦʽΪ��_______________________��
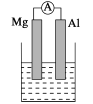
(2)���ձ�����ҺΪ����������Һ����Ϊ________(��Mg��Al)���ܷ�Ӧ��ѧ����ʽΪ________________________________��
��. (3)��Al��Cu��Ũ�������ԭ��أ��为���ĵ缫��ӦʽΪ______________________��
��.�й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�(CH3OH)ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��
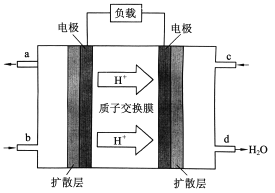
(4)�õ�ع���ʱ��b��ͨ�������Ϊ_______��c��ͨ�������Ϊ______��
(5)�õ�������ĵ缫��ӦʽΪ��_____________��
���𰸡�Mg���ܽ⣬AlƬ��������ð����������ָ��ƫת Mg��2e�� = Mg2+ Al 2Al+2NaOH+2H2O = 2NaAlO2+3H2�� Cu��2e�� = Cu2+ CH3OH O2����� O2+4e��+4H+ = 2H2O
��������
��.��1��þ�������ã����ձ�����ҺΪϡ���ᣬþΪ��������Ϊ������
��2������������������Һ��Ӧ�����ձ�����ҺΪ����������Һ����Ϊ������þΪ������
��. ��3����Al��Cu��Ũ�������ԭ��أ������淢���ۻ�����ͭ��Ũ���ᷢ��������ԭ��Ӧ�����ԭ����и��ӷ���ʧ���ӵ�������Ӧ����
��. ��4�������������ƶ�����֪���Ҳ�缫Ϊ���������缫Ϊ������������ͨ��ȼ�ϣ�
��5���������������õ��ӷ�����ԭ��Ӧ��
��. (1)þ��������,���ձ�����ҺΪϡ����,�γ�ԭ��ط�Ӧ,þΪ����,��Ϊ����,��������Mg2e=Mg2+���ɹ۲쵽Mg���ܽ⣬AlƬ��������ð����������ָ��ƫת��
�ʴ�Ϊ��Mg���ܽ⣬AlƬ��������ð����������ָ��ƫת��Mg2e=Mg2+��
(2)����������������Һ��Ӧ,���ձ�����ҺΪ����������Һ,��Ϊ������þΪ����,��������NaAlO2������������������Ӧ���ܷ���ʽΪ2Al+2NaOH+2H2O = 2NaAlO2+3H2����
�ʴ�Ϊ��Al��2Al+2NaOH+2H2O = 2NaAlO2+3H2����
��. ��3����Al��Cu��Ũ�������ԭ��أ�����������ͭ������������ʧ���ӵ�������Ӧ����ͭ���ӣ���缫��ӦʽΪCu��2e�� = Cu2+��
��. ��4�����������ƶ�����֪�Ҳ�缫Ϊ������c��ͨ���������缫Ϊ������b��Ϊ������ͨ��ȼ�ϼ״����ʴ�Ϊ��CH3OH��O2�������
(2)�����������õ��Ӻ������ӷ�Ӧ����ˮ���缫��ӦʽΪ��O2+4e��+4H+ = 2H2O��

����Ŀ�����г�ȥ���ʵķ����������
���� | ���� | ���Ӽ� | |
A | þ�� | ���� | �����ռ���Һ |
B | FeCl3(��Һ) | FeCl2 | ���� |
C | CO2 | HCl | ����Na2CO3��Һ |
D | Cl2 | HCl | ����ʳ��ˮ |
A.AB.BC.CD.D
����Ŀ����1����һ���¶��£��ڹ̶�������ܱ������н��п��淴Ӧ��N2(g)��3H2(g)![]() 2NH3(g)���ÿ��淴Ӧ�ﵽƽ��ı�־��________��
2NH3(g)���ÿ��淴Ӧ�ﵽƽ��ı�־��________��
A��3v��(H2)��2v��(NH3) B����λʱ������m mol N2��ͬʱ����3m mol H2
C�������ڵ���ѹǿ������ʱ����仯 D�����������ܶȲ�����ʱ��仯
��2����ҵ�Ͽ�����Ȼ��Ϊԭ������ȡ�ϳɰ���ԭ����������ij�о���ѧϰС���ͬѧģ�ҵ��ȡ������ԭ������һ���¶��£����Ϊ2 L�ĺ����ܱ������в�����±���ʾ���ݡ���ش��������⣺
ʱ��/min | CH4/mol | H2O/mol | CO/mol | H2/mol |
0 | 0.40 | 1.00 | 0 | 0 |
5 | a | 0.80 | c | 0.60 |
7 | 0.20 | b | 0.20 | d |
�����������ݣ��ж�5 minʱ��Ӧ�Ƿ���ƽ��״̬��______(��ǡ���)��ǰ5 min��Ӧ��ƽ����Ӧ����v(CH4)��____________________��
��3�����º����£���2 mol ����A��2 mol����Bͨ�����Ϊ2L���ܱ������У��������·�Ӧ��2A(g)��B(g) xC(g)��2D(s)��2 min��Ӧ�ﵽƽ��״̬����ʱʣ��1.2 mol B�������C��Ũ��Ϊ1.2 mol/L��
��x��________��
��A��ת������B��ת����֮��Ϊ________��