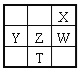
��Ŀ����
����Ŀ����ʯӢ����̿Ϊԭ�ϵIJ��ֻ���������ͼ��ʾ��

�ش��������⣺
(1)��ӦI�ڹ�ҵ�ϵ������������ֹ裬Ϊ������ƷR���Ƿ������������ʣ�ȡ����R����ϡ�����ܽ⣬ȡ�ϲ���Һ�����ټ�����Լ���______������ĸ����
a����ˮ b����ˮ c��KSCN��Һ d��NaCl��Һ
(2)��Ӧ��Ҫ�ڸ��ﻷ���±��У���ԭ����______���������õ���SiHCl3���е�Ϊ33.0�棩�к�������SiCl4�е�Ϊ57.6�棩��HC1���е�Ϊ- 84.7�棩�����ᴿSiHCl3�ķ���Ϊ______________��
(3)��ˮ����ͨ�����ȵ�ú�ۿɲ���ˮú��������Ҫ�ɷ���CO��H2����ҵ�Ͻ�ˮú��Һ�����Ƶõ�CH3OH�к��еĻ�ѧ������Ϊ________������Ӽ������ۼ�����������ͼ�еķ�Ӧ��Ϊ������ԭ��Ӧ���仯ѧ����ʽΪ_______________��
���𰸡� bc ��ֹ���ɵ�SiHCl3��ˮ��Ӧ ���� ���ۼ� SiHCl3+(n+2)H2O =SiO2��nH2O+ H2��+3HC1��
��������(1)���ƷR�к������������ʣ���ϡ�����ܽ����ϲ���Һ�к���Fe2+������ʱ��Ҫ�ȵμ�KSCN��Һ����ɫ���ٵμ���ˮ���ɫ���ʴ�Ϊbc��
(2)SiHCl3��ˮ�⣬��Ӧ��Ҫ�ڸ��ﻷ���½��У�Ŀ�ĵ�Ϊ�˷�ֹ���ɵ�SiHCl3��ˮ��Ӧ��SiHCl3��SiCl4���ڷе���죬������������������з����ᴿ��
(3)CH3OH�ǹ��ۻ����ֻ���й��ۼ�������ͼ�еķ�Ӧ��Ϊ������ԭ��Ӧ������SiHCl3��HԪ��Ϊ-1�ۣ��������ͼ�з�Ӧ������������ݵ����غ��ԭ���غ㼴�ɵõ���Ӧ����ʽΪSiHCl3+(n+2)H2O =SiO2��nH2O+ H2��+3HC1����

����Ŀ����ij�¶��£���������ʼ�����Ϊ1L���ܱ������а��±���ʾͶ�ϣ�������Ӧ��2SO2(g)+O2(g) ![]() 2SO3(g)��H��0���ﵽƽ���������˵����ȷ����
2SO3(g)��H��0���ﵽƽ���������˵����ȷ����
������� | �������� | ��ʼ���ʵ���/mol | ƽ��ʱSO3�� ���ʵ���/mol | ||
SO2 | O2 | SO3 | |||
�� | ���º��� | 2 | 1 | 0 | 1.2 |
�� | ���Ⱥ��� | 0 | 0 | 2 | a |
�� | ���º�ѹ | 2 | 1 | 0 | b |
A. ƽ��ʱSO3�����ʵ�����a��1.2��b��1.2
B. ��������ƽ�ⳣ����ͬ
C. ����I��SO2��ת��������������SO3��ת����֮��С��1
D. ����ʼʱ���������г���1.0molSO2(g)��0.40molO2(g)��1.40molSO3(g)�����ʱV����V��