��Ŀ����
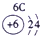
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA |
| 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A |
|
| 2 |
|
|
| �� | �� | �� |
|
|
| 3 | �� |
| �� | �� |
|
| �� |
|
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_____________________��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
��4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ�����ĵ���ʽ�� ��
��������ݵ�ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ
��5��������ݵ�����������ˮ���ﷴӦ�����ӷ���ʽΪ
��
��1���ܣ��ݣ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_ _ Na>Al>O________________��
��2���ڣ��ۣ��ߵ���ۺ������������ǿ������˳����HNO3>H2CO3>H2SiO3��H4SiO4����
��3���٣��ܣ��ݣ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��__NaOH�ĵ���ʽ��_��_��______________��
��4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ�����ĵ���ʽ��CO2�ĵ���ʽ
![]() ��������ݵ�ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ
��������ݵ�ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ
2Mg+ CO2 ==2MgO+C
��5��������ݵ�����������ˮ���ﷴӦ�����ӷ���ʽΪ
2Al+2OH-+2H2O==2AlO2-+3H2��
����:��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�







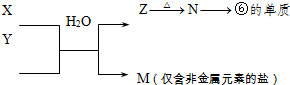
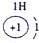
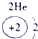
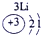
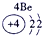
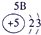
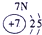
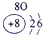
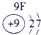
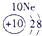
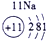





 ��ʾ����
��ʾ����