��Ŀ����
����Ŀ������(O2)�ͳ���(O3)����Ԫ�ص�����ͬ�������壬��֪�Ȼ�ѧ����ʽ��
4Al(s)��3O2(g)===2Al2O3(s)����H1
4Al(s)��2O3(g)===2Al2O3(s)����H2
3O2(g)===2O3(g)����H3��(����)
A����H1����H2����H3
B����H1����H2����H3
C����H2����H1����H3
D����H2����H1����H3��0
���𰸡�A
����������1���Ȼ�ѧ����ʽ��ȥ��2���Ȼ�ѧ����ʽ������3O2(g)===2O3(g)����H3����H1����H2����A��ȷ��

 С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�����Ŀ���й�Ԫ��X��Y��Z��D��E����Ϣ���£�
Ԫ�� | �й���Ϣ |
X | Ԫ����Ҫ���ϼۣ�2��ԭ�Ӱ뾶Ϊ0.0074 nm |
Y | ��������������������������֮��Ϊ4 |
Z | ������X�ĵ�����ȼ�գ����������������������֮һ |
D | ����������Ӧ��ˮ�����ܵ������������ȵ����������� |
E | �����������еij�������������Ʒ�ڳ�ʪ�������ױ���ʴ���� |
��ش���������(�û�ѧ�����ʾ)��
��1��X��һ���⻯�������ʵ������ȡX�ĵ��ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ____________��
��2���Ƚ�Y��Z���⻯����ȶ���________(�û�ѧʽ��ʾ)��
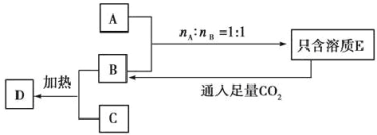
��3��EԪ����YԪ�ؿ��γ�EY2��EY3���ֻ����������˵������ȷ����________(�����)
��ͨ��ʵ��������EY3��Һʱ����ֱ����ˮ�ܽ�EY3����
��EY2����ͨ������ֱ�ӻ��ϲ���
��ͭƬ��̼����EY3��Һ���ԭ��أ�������ͭƬ�ص�������̼��
��Y��Z��D�����Ӱ뾶��С���μ�С
��4��Y�����������Ϊ��ɫҺ�壬��0.25 mol��������һ������ˮ��ϵõ�һ��ϡ��Һʱ���ų�Q kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ_______________________________��
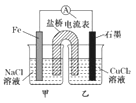
��5��д��E�ڳ�ʪ�Ŀ����з�����ʴʱ�����ϵĵ缫��Ӧʽ_____________________��