��Ŀ����
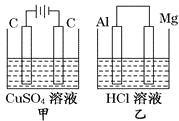
����Ŀ������������Ԫ��X��Y��Z��W��ԭ������������������Ԫ���γɵĵ�������Ϊm��n��p��q����ЩԪ����ɵĶ�Ԫ������r��t��u������uΪ�γ��������Ҫ����֮һ��25��ʱ��0.01mol/L��v��Һ��pH=12���������ʵ�ת����ϵ��ͼ��ʾ������˵����ȷ����

A. ԭ�Ӱ뾶�Ĵ�С:W>Z>Y>X
B. v������ˮ�ĵ����u�ܴٽ�ˮ�ĵ���
C. ճ��q���Թܿ��þƾ�ϴ��
D. Z�ֱ���Y��W��ɵĻ������л�ѧ�����Ϳ�����ͬ
���𰸡�D
������������������������Ԫ��X��Y��Z��W��ԭ������������������Ԫ���γɵĵ�������Ϊm��n��p��q��r��t��u����ЩԪ����ɵĶ�Ԫ���������uΪ�γ��������Ҫ����֮һ��uΪSO2��25��ʱ��0.01mol/L��v��Һ��pH=12����vΪNaOH�����ͼ��ת����֪��mΪH2��nΪO2��pΪNa��rΪH2O��tΪNa2O2����X��Y��Z��W�ֱ�ΪH��O��Na��S��qΪS���ʣ��Դ˽�������
��⣺������������֪��X��Y��Z��W�ֱ�ΪH��O��Na��S��mΪH2��nΪO2��pΪNa��rΪH2O��tΪNa2O2��vΪNaOH��qΪS���ʣ�uΪSO2��A�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴ�������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶�Ĵ�С��X��Y��W��Z��ѡ��A����B��uΪSO2����ˮ��Ӧ���������ᣬ��Һ�����ԣ�����ˮ�ĵ��룬ѡ��B����B��Ԫ�صķǽ�����ΪO��S��H����Y��W��X����B��ȷ��C��qΪS���ʣ����ھƾ�����ճ��q���Թܲ����þƾ�ϴ�ӣ�Ӧ��Ũ������������Һ��ѡ��C����D��Z�ֱ���Y��W��ɵĻ�����Na2O��NaH�л�ѧ�����;�Ϊ���Ӽ�����ͬ��ѡ��D��ȷ����ѡD��

 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�����Ŀ����֪:H2(g)+I2(g)![]() 2HI(g)��H=-14.9kJ��mol-1��ij�¶��£��������Ϊ2.0L�ļס������������ܱ������г��뷴Ӧ�����ʼ���ʵ������±���ʾ�����з�Ӧ�ﵽƽ��ʱ�����c(H2)=0.008mol��L-1�������ж���ȷ���ǣ� ��
2HI(g)��H=-14.9kJ��mol-1��ij�¶��£��������Ϊ2.0L�ļס������������ܱ������г��뷴Ӧ�����ʼ���ʵ������±���ʾ�����з�Ӧ�ﵽƽ��ʱ�����c(H2)=0.008mol��L-1�������ж���ȷ���ǣ� ��
��ʼ���ʵ��� | n(H2)/mol | n(I2)/mol | n(HI)/mol |
�� | 0.02 | 0.02 | 0 |
�� | 0.04 | 0.04 | 0 |
A. ƽ��ʱ������H2��ת�����Ǽ��е�2��
B. ƽ��ʱ�����л�������ɫ��������
C. ƽ��ʱ���ס����������ı仯ֵ���
D. ���¶��£���Ӧ��ƽ�ⳣ��K=0.25