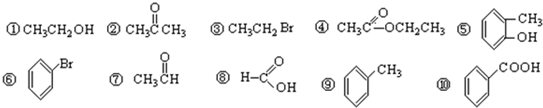
��Ŀ����
16���±���Ԫ�����ڱ���һ���֣�����Ա��еĢ١����Ԫ�ػش����⣮���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | ||||
| 4 | �� |
 ��
����2������õĽ���Ԫ����K����Ԫ�ط��ţ���ͬ������ѧ�������ȶ���Ԫ����Ne��
��3����������Ԫ���γɵ���̬�⻯���У����ȶ����⻯����HCl���ѧʽ����
��4���ٵ�����������л�ѧ�����������ڹ��ۼ���
��5���õ���ʽ��ʾ�������ɵ�A2B�ͻ�������γɹ��̣�
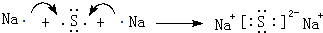 ��
����6���ݵ����������������������������������Ʒ�Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
��7���ڵĵ�����H2��Ӧ�Ļ�ѧ����ʽΪN2+3H2$\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$2NH3��
���� ����Ԫ�������ڱ��е����λ�ÿ�֪������C������N������O������Na������Al������S������Cl������Ne������K��
��1������Cl��ԭ�Ӻ�����17�����ӣ���3�����Ӳ㣬���������Ϊ2��8��7��
��2��ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ��ϡ�����廯ѧ�������ȶ���
��3��ͬ����������ҷǽ�������ǿ���ǽ�����Խǿ���⻯��Խ�ȶ���
��4���ٵ����������ΪCO2��������̼�����к�������̼��˫����Ϊ���ۻ����
��5���������ɵ�A2B�ͻ�����ΪNa2S�����������������ӹ��ɣ�
��6���ݵ����������ΪAl2O3��������������������������Ʒ�Ӧ����ƫ��������ˮ��
��7��������H2�ڸ��¸�ѹ�����������·�Ӧ����������
��� �⣺����Ԫ�������ڱ��е����λ�ÿ�֪������C������N������O������Na������Al������S������Cl������Ne������K��
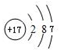
��1������Cl��ԭ�Ӻ�����17�����ӣ���3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ������Ԫ����K�Ľ�������ǿ��ϡ������Neԭ�������Ϊ�ȶ��ṹ����ѧ�������ȶ����ʴ�Ϊ��K��Ne��
��3��ͬ����������ҷǽ�������ǿ���ʵ���������Cl�ķǽ�������ǿ���ǽ�����Խǿ���⻯��Խ�ȶ�����HCl���ȶ����ʴ�Ϊ��HCl��
��4���ٵ����������ΪCO2�����й��ۼ����ʴ�Ϊ�����ۼ���
��5���������ɵ�A2B�ͻ�����ΪNa2S�����������������ӹ��ɣ��õ���ʽ��ʾ�����γɹ���Ϊ��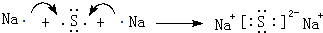 ���ʴ�Ϊ��
���ʴ�Ϊ��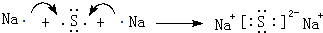 ��
��
��6���ݵ����������ΪAl2O3��������������������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ������Al2O3+2OH-=2AlO2-+H2O��
��7��������H2�ڸ��¸�ѹ�����������·�Ӧ������������Ӧ����ʽΪ��N2+3H2$\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$2NH3���ʴ�Ϊ��N2+3H2$\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$2NH3��
���� ���⿼��Ԫ�����ڱ���Ԫ��������Ӧ�ã��ѶȲ���ע�������õ���ʽ��ʾ��ѧ�������ʵ��γɣ�

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�| ��ѧ�� | Si-O | Si-Cl | H-H | H-Cl | Si-Si | Si-C | Cl-Cl |
| ����/kJ•mol-1 | 460 | 360 | 436 | 431 | 176 | 347 | 243 |
| A�� | �������ȶ��Ĺ��ۼ���Si-Si | |
| B�� | Cl2��g����2��Cl��g������H=-243��kJ•mol | |
| C�� | H2����g��+Cl2��g��=2HCl��g������H=-183��kJ•mol | |
| D�� | ���ݱ��������ܼ����SiCl4��g��+2��H2��g���TSi��s��+4��HCl��1���ġ�H |
| A�� | �����ķϵ�ز��������Ⱦ | |
| B�� | п�̸ɵ�ظ���п�ڹ���ʱʧȥ���� | |
| C�� | ����ȼ�ϵ���ǽ�����ֱ��ת��Ϊ���� | |
| D�� | ���ε�صij������ǽ���ѧ��ת���ɵ��ܵĹ��� |
���¶Ⱥ����һ����ijһ����Ũ�Ȳ��ٱ仯ʱ ���¶Ⱥ����һ����������ѹǿ���ٱ仯ʱ
������һ������������ƽ����Է����������ٱ仯ʱ ���¶Ⱥ����һ��������������ɫ���ٱ仯ʱ ���¶Ⱥ�ѹǿһ�������������ܶȲ��ٱ仯ʱ ��2v����NO2��=v����N2O4��
�ߵ�λʱ��������2x mol NO2��ͬʱ����x mol N2O4��
| A�� | �٢ڢۢܢ� | B�� | �ڢۢ� | C�� | �٢ڢۢ� | D�� | �ۢܢݢ� |
| A�� | 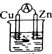 �Ҵ� | B�� |  ϡH2SO4 | C�� | 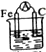 ϡHCl | D�� | 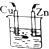 ϡH2SO4 |
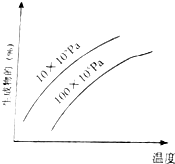 �ɿ��淴Ӧ����ͼ����ͼ��������Ϊ��������ƽ�������еİٷֺ��������жԸ÷�Ӧ���ж���ȷ���ǣ�������
�ɿ��淴Ӧ����ͼ����ͼ��������Ϊ��������ƽ�������еİٷֺ��������жԸ÷�Ӧ���ж���ȷ���ǣ�������| A�� | ��Ӧ����һ�������� | B�� | �������в�һ�������� | ||
| C�� | ����Ӧһ���Ƿ��ȷ�Ӧ | D�� | ����Ӧһ�������ȷ�Ӧ |
��1���л������̼Ԫ�أ�����Ϊ����л�����������ȫȼ�պ�IJ������õ��Լ�Ϊ����ʯ��ˮ����ˮ����ͭ�����Լ����ƣ���
��2����Ϊ���ǽ������ϵ����ǣ�д�����������һ�ֹ�ҵ��;�ƹ��ά��
��3���������ӣ�Sn2+����Fe3+��Ӧ����Sn4+����Ӧ�����ӷ���ʽΪSn2++2Fe3+=Sn4++2Fe2+
��4��PbO2Ϊ��ɫ��ĩ�����¶������ֽ⣺PbO2��PbxOx��PbO��PbxOy��ʾ��ͬ�¶���Ǧ��������Ļ�ѧʽ����
��֪��23.90gPbO2�ڲ�ͬ�¶��²������壨��Ϊ���������������
| �¶�/�� | T1 | T2 | T3 | T4 |
| ������������/g | 23.90 | 23.10 | 22.94 | 22.30 |
| A�� | Na+��Cu2+��Br-��Cl- | B�� | K+��NH4+��Cl-��Na+ | ||
| C�� | K+��Na+��NO3-��H+ | D�� | K+��S2-��SO42-��OH- |