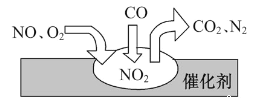
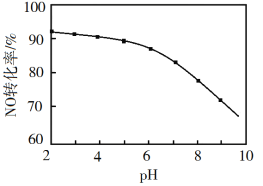
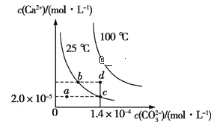
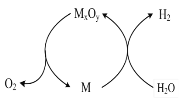
��Ŀ����
����Ŀ����1����ͼΪ�ɱ��ľ���ṹʾ��ͼ��
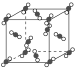
ͨ���۲������ÿ��CO2������Χ���ڵȾ����CO2������__������CO2���������ʵ㣬�辧���߳�Ϊapm������ڵ�����CO2���ӵľ���Ϊ__pm��
��2���ڱ������У�ˮ����֮�����Ҫ��������__������__�����ڸ���Ҫ�������빲�ۼ�һ������__�ԣ���1��ˮ������Χֻ��__�����ڵ�ˮ���ӣ���Щˮ����λ��__�Ķ��㡣�������з�ʽʹ��������ˮ���ӵĿռ�������__(�����ϸ��������ϵ���)���ʱ����ܶȱ�ˮ���ܶ�Ҫ__(������������С��)��
���𰸡�12 ![]() a ��� ���»��� ���� 4 ������ �ϵ� С
a ��� ���»��� ���� 4 ������ �ϵ� С
��������
�ɱ��Ƿ��Ӿ��壬CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ�����������������CO2���ӷֲ�����ö���������12����������ϣ�������з����Ժͱ����ԡ�
��1���ɱ��Ƿ��Ӿ��壬CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ�����������������CO2���ӷֲ�����ö���������12����������ϣ����Ըɱ������У�ÿ��CO2������Χ��12����֮�����ҵȾ��CO2���ӣ����ڵ�����CO2���ӵľ���Ϊ��Խ��ߵ�һ�룬�����Ϊ![]() a pm��
a pm��
(2)�����Ԫ��֮����γ���������ڱ������У�ˮ����֮�����Ҫ�������������ͬʱ���з��»���������빲�ۼ�һ�����з����ԣ�ͬʱ��������б����ԣ���1��ˮ������Χֻ��4�����ڵ�ˮ���ӣ�����Щˮ����Ϊ���㹹��һ���������壻��������ķ����ԣ�����֮��Ŀ�϶�ϴʱ�������ˮ���ӵĿռ������ʽϵͣ��������ۻ�ʱ��������ƻ���ˮ���Ӽ�Ŀ�϶��С���ܶ����ʱ����ܶȱ�ˮ���ܶ�ҪС��

 �ŵ������ϵ�д�
�ŵ������ϵ�д�