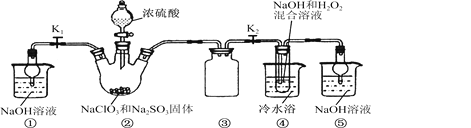
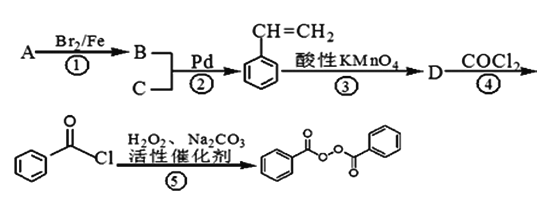
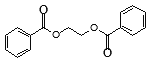
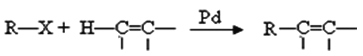��Ŀ����
����Ŀ��t��ʱ��ijNaOHϡ��Һ��c(H��)=10a mol/L��c(OH)=10b mol/L����֪a��b=12����ش��������⣺
��1�����¶���ˮ�����ӻ�����KW=__________________��
��2��NaOH�����ʵ���Ũ��Ϊ_________________����ˮ�������c(OH)Ϊ______________��
��3������NaOH��Һ���£�pH________(���������������С������������)��
��4�����¶��£���pH=3��H2SO4��pH=11��NaOH�������ϣ����Ϻ���Һ��pH=____(��֪lg2=0.3) ��������Һ����仯��
���𰸡� 10-12 10b mol/L 10amol/L ��� 10.7
��������(1)��Һ�е����ӻ�Kw=c(H+)��c(OH-)=10-a��10-b=10-(a+b)=10-12���ʴ�Ϊ��10-12��
(2)��NaOH��Һ��NaOH�����ʵ���Ũ��Ϊc(NaOH)=c(OH-)=10-bmol/L��������Һ�е����ӻ�Kw=c(H+)��c(OH-)=10-12����NaOH��Һ����ˮ�������c(OH-)Ϊ10-a���ʴ�Ϊ��10-bmol/L��10-amol/L��
(3)����NaOH��Һ����������ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�������С����Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ�ȼ�С��������ҺpH�������ʴ�Ϊ�������
(4)���¶��£�pH=3��H2SO4��c(H��)=103mol/L��pH=11��NaOH��c(H��)=1011 mol/L����c(OH)=![]() =101mol/L���������ϣ����������ӹ�������Һ�Լ��ԣ���Ϻ���Һ��c(OH)=
=101mol/L���������ϣ����������ӹ�������Һ�Լ��ԣ���Ϻ���Һ��c(OH)=![]() ��0.05mol/L��c(H��)=
��0.05mol/L��c(H��)= ![]() mol/L=2��1010mol/L��pH= -lg(2��1010)=10.7���ʴ�Ϊ��10.7��
mol/L=2��1010mol/L��pH= -lg(2��1010)=10.7���ʴ�Ϊ��10.7��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ������HCl����״��������ƿ������Ȫʵ���õ���ϡ������Һ���ñ�������������Һ�ζ�����ȷ����ϡ�����ȷ���ʵ���Ũ�ȡ��ش��������⣺
��1���õζ�ʵ��ʢװ��Һ��������_______________������������ʱӦ����__________mL����������������Ϊ50mL����Һ��Ϊ0����������������Һ��ų�����ų�����Һ���_______50mL������������������������������
��2�����õζ�ʵ���÷�̪��ָʾ�����ﵽ�ζ��յ�ʱ����Һ��ɫ��____ɫ��Ϊ____ɫ�ұ���30s�ڲ���ɫ��
��3���������ֲ�ͬŨ�ȵı�����������Һ������Ϊ����ʵ��ǵ�______�֡�
��5.000 mol/L ��0. 5000 mol/L ��0.0500 mol/L
��4���������������ʵı�����������Һ�ζ�ϡ���ᣬ��������������ζ����ʵ���������£�
ʵ���� | ��������������mL�� | ��������������Һ�������mL�� |
1 | 20.00 | 17.30 |
2 | 20.00 | 17.02 |
3 | 20.00 | 16.98 |
���õ�ϡ��������ʵ���Ũ��Ϊ_________________________��
��5�����в������µĽ��������ƫ������ƫС������Ӱ������
�ٵζ���ϴ����ֱ��װ�������������Һ���еζ���___________
�ڵζ�ǰ����ʱ���ӣ��ζ������ʱ���ӣ�___________
�����ú���Na2O���ʵ��������ƹ������Ʊ���Һ��___________
�ܵζ�ǰ����ʽ�ζ��������ݣ��ζ�����ʧ��___________��