��Ŀ����
����Ŀ���ȼ��仯������;�㷺������NaCl��HCl��������Ҫ�ĺ��Ȼ����
��1��NaCl�ĵ���ʽΪ___��HCl�ĵ���ʽ___��
��2����ҵ�����������ķ����ǵ�ⱥ��ʳ��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ��___�����ʱ������___���õ�������___����������ڡ�
��3��ʵ�����Ʊ��Ȼ�������Ļ�ѧ����ʽΪ___��
��4����ͼΪH2��Cl2ȼ�յķ�Ӧ�����仯ʾ��ͼ������ݴ�ͼд���÷�Ӧ���Ȼ�ѧ����ʽ��___��

���𰸡�![]()
![]()
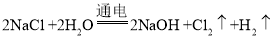 �� ʪ��ĵ��۵⻯����ֽ
�� ʪ��ĵ��۵⻯����ֽ ![]() H2(g)+Cl2(g)=2HCl(g) ��H= -184.6kJ/mol
H2(g)+Cl2(g)=2HCl(g) ��H= -184.6kJ/mol
��������
��1��NaCl�����ӻ����HCl�ǹ��ۻ�������ݵ���ʽ����д���������д��
��2����ҵ�ϵ�ⱥ��ʳ��ˮʱ����������������ʧ���ӵ�������Ӧ���缫��ӦʽΪ��2Cl--2e-=Cl2��������ʪ��ĵ��۵⻯�ر������������������������������ˮ����������ӷ����õ��ӵĻ�ԭ��Ӧ���缫��ӦʽΪ��2H2O+2e-=H2+2OH-��
��3��ʵ������Ũ�������Ȼ��ƹ��干���Ʊ��Ȼ������壻
��4����ͼ��֪1mol H2�����1mol Cl2�������������2mol HCl�����������184.6kJ���÷�ӦΪ���ȷ�Ӧ���ݴ˽��
��1��NaCl�����ӻ���������ʽΪ![]() ��HCl�ǹ��ۻ���������ʽ
��HCl�ǹ��ۻ���������ʽ![]() ��
��
��2����ҵ�ϵ�ⱥ��ʳ��ˮʱ����������������ʧ���ӵ�������Ӧ���缫��ӦʽΪ��2Cl--2e-=Cl2��������ʪ��ĵ��۵⻯�ر������������������������������ˮ����������ӷ����õ��ӵĻ�ԭ��Ӧ���缫��ӦʽΪ��2H2O+2e-=H2+2OH-����÷�Ӧ�ܵĻ�ѧ����ʽΪ��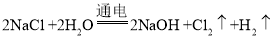 �����ʱ�����������õ�������ʪ��ĵ��۵⻯����ֽ����������ڣ�
�����ʱ�����������õ�������ʪ��ĵ��۵⻯����ֽ����������ڣ�
��3��ʵ������Ũ�������Ȼ��ƹ��干���Ʊ��Ȼ������壬��Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
��4����ͼ��֪1mol H2�����1mol Cl2�������������2mol HCl�����������184.6kJ���÷�ӦΪ���ȷ�Ӧ����÷�Ӧ���Ȼ�ѧ����ʽΪ��H2(g)+Cl2(g)=2HCl(g) ��H= -184.6kJ/mol��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ��(1)��ӦFe(s)��CO2(g)![]() FeO(s)��CO(g)����H1��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)
FeO(s)��CO(g)����H1��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)![]() FeO(s)��H2(g)����H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)��H2(g)����H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
700 �� | 900 �� | |
K1 | 1.47 | 2.15 |
K2 | 2.38 | 1.67 |
�ٷ�ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)����H��ƽ�ⳣ��ΪK������H��________(����H1����H2��ʾ)��K��________(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g)
CO(g)��H2O(g)����H��ƽ�ⳣ��ΪK������H��________(����H1����H2��ʾ)��K��________(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)��_____________________��Ӧ(��������������������)��
CO(g)��H2O(g)��_____________________��Ӧ(��������������������)��
�����ж�CO2(g)��H2(g)![]() CO(g)��H2O(g)�ﵽ��ѧƽ��״̬��������_______(����ĸ)��
CO(g)��H2O(g)�ﵽ��ѧƽ��״̬��������_______(����ĸ)��
A��������ѹǿ���䡡�� B�����������c(CO)����
C��v��(H2)��v��(H2O) D��c(CO)��c(CO2)
(2)һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������Ӧ��Fe(s)��CO2(g)![]() FeO(s)��CO(g)����H>0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)��CO(g)����H>0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��

�ٸ������·�Ӧ��ƽ�ⳣ��Ϊ______��������������CO2����ʼŨ��Ϊ2.0 mol��L��1����ƽ��ʱCO2��Ũ��Ϊ______mol��L��1��
�����д�ʩ����ʹƽ��ʱ �������________(����ĸ)��
�������________(����ĸ)��
A�������¶� B������ѹǿ
C������һ������CO2 D���ټ���һ��������
����Ŀ�������ĸ��Թ���,��������ӦZn+2HCl=ZnCl2+H2��������H2�ķ�Ӧ������С����
�Թ� | ����Ũ��(mol/L) | �¶�(��) | п��״̬ |
A | 0.5 | 20 | ��״ |
B | 0.5 | 20 | ��ĩ״ |
C | 2 | 35 | ��״ |
D | 2 | 35 | ��ĩ״ |
A. AB. BC. CD. D



