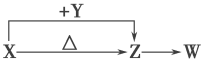
��Ŀ����
����Ŀ����ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��100 g 16.0%�ĵ�CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��
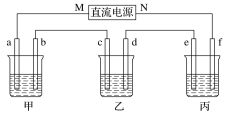
��ͨ��Դ������һ��ʱ���ñ���K2SO4����������Ϊ12.20%������c�缫�������ӡ��ݴ˻ش����⣺
��1����Դ��N��Ϊ________����
��2���缫b�Ϸ����ĵ缫��ӦʽΪ____________________________________________
��3���缫b�����ɵ������ڱ�״���µ����_________________��
��4���缫c�������仯��________g��
���𰸡���4OH-��4 e-=2H2O+O211.2L64
��������
��1������C�缫�������ӣ���c�������ķ�ӦΪ��Cu2++2e-=Cu����C��Ϊ�������ɴ˿��Ƴ�bΪ������aΪ������MΪ������NΪ������
��2������ΪNaOH���൱�ڵ��H2O������b��Ϊ������OH-�ŵ磬��4OH--4e-=2H2O+O2����
��3������ת�Ƶ��ӵ�������ϵ缫��Ӧʽ���м��㼴�ɣ�
��4�����Cu2++2e-=Cu��Ӧ������ת�Ƶ��ӵ���������ͭ��������
��1���ұ���c�������ӣ�˵��Cu������c�缫�ϣ������Ǵ�b-c�ƶ���M�Ǹ�����NΪ��������ˣ�������ȷ���ǣ�����
��2������ΪNaOH���൱�ڵ��H2O������b��Ϊ������OH-�ŵ磬��4OH--4e-=2H2O+O2������ˣ�������ȷ���ǣ�4OH--4e-=2H2O+O2����
��3������ΪK2SO4���൱�ڵ��ˮ�������ˮ������Ϊx���ɵ��ǰ��������������У�100��10%=��100-x����12.2%����x=18g����Ϊ1mol���ɷ���ʽ2H2+O2�T2H2O����֪��������2molH2O��ת��4mol���ӣ�����������Ӧ��ת��2mol���ӣ�������O2Ϊ2��1/4=0.5mol������µ����Ϊ0.5��22.4=11.2L����ˣ�������ȷ���ǣ�11.2L��
��4��������·�Ǵ����ģ�����ÿ���ձ��еĵ缫��ת�Ƶ���������ȵģ����ݵ缫��Ӧ��Cu2++2e-=Cu������֪��ת��2mol�������ɵ�m��Cu��=64��1=64g����ˣ�������ȷ���ǣ�64��

����Ŀ���������ӷ���ʽ����д�����۾���������( )
ѡ�� | ���ӷ���ʽ | ���� |
A | ��2 mol Cl2ͨ�뺬1 mol FeI2����Һ�У� 2Fe2����2I����2Cl2===2Fe3����4Cl����I2 | ��ȷ��Cl2�������ɽ�Fe2����I�������� |
B | Ba(HCO3)2��Һ��������NaOH��Һ��Ӧ�� Ba2����HCO3-��OH��===BaCO3����H2O | ��ȷ����ʽ����Ӧ�������κ�ˮ |
C | ����SO2ͨ��NaClO��Һ�У� SO2��H2O��ClO��===HClO��HSO3- | ��ȷ��˵�����ԣ�H2SO3ǿ��HClO |
D | 1 mol/L��NaAlO2��Һ��2.5 mol/L��HCl��Һ�������ϣ� 2AlO2-��5H��===Al3����Al(OH)3����H2O | ��ȷ����һ����Ӧ�͵ڶ�����Ӧ���ĵ�H�������ʵ���֮��Ϊ2��3 |
A. A B. B C. C D. D