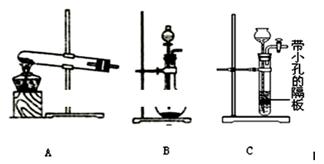
��Ŀ����
(12��).
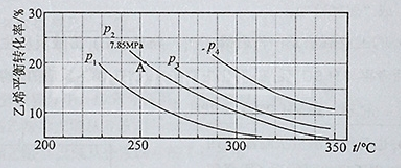
��1.0 L�ܱ������з���0.10molA(g)����һ���¶Ƚ������·�Ӧ:
A(g) B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ��
B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ��
��Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±���
�ش���������:
��1�������A��ƽ��ת���ʣ�Ӧ��ȡ�Ĵ�ʩΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2������ѹǿP����ʼѹǿP0���㷴Ӧ��A��ת���ʦ�(A)�ı���ʽΪ�ߣߣߣߣߣߣߡ�ƽ��ʱA ��ת����Ϊ�ߣߣߣߣ�.
��3�� ������ѹǿp����ʼѹǿp0��ʾ��Ӧ��ϵ�������ʵ���n���ͷ�Ӧ��A�����ʵ���n��A����
n����_______mol��n��A����_______mol��
���±�Ϊ��Ӧ��AŨ���뷴Ӧʱ������ݣ�����a�� _______________
�����÷�Ӧ�з�Ӧ���Ũ��c��A���仯��ʱ��������t���Ĺ��ɣ��ó��Ľ����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ��ɴ˹����Ƴ���Ӧ��12hʱ��Ӧ���Ũ��c��A��Ϊ_______mol��L-1
��1.0 L�ܱ������з���0.10molA(g)����һ���¶Ƚ������·�Ӧ:
A(g)
 B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ��
B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ����Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±���
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100kPa | 4.91 | 5.58 | 6.32 | 7.31 | 8.54 | 9.50 | 9.52 | 9.53 | 9.53 |
�ش���������:
��1�������A��ƽ��ת���ʣ�Ӧ��ȡ�Ĵ�ʩΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2������ѹǿP����ʼѹǿP0���㷴Ӧ��A��ת���ʦ�(A)�ı���ʽΪ�ߣߣߣߣߣߣߡ�ƽ��ʱA ��ת����Ϊ�ߣߣߣߣ�.
��3�� ������ѹǿp����ʼѹǿp0��ʾ��Ӧ��ϵ�������ʵ���n���ͷ�Ӧ��A�����ʵ���n��A����
n����_______mol��n��A����_______mol��
���±�Ϊ��Ӧ��AŨ���뷴Ӧʱ������ݣ�����a�� _______________
| ��Ӧʱ��t/h | 0 | 4 | 8 | 16 |
| C��A��/��mol��L-1�� | 0.10 | a | 0.026 | 0.0065 |
�����÷�Ӧ�з�Ӧ���Ũ��c��A���仯��ʱ��������t���Ĺ��ɣ��ó��Ľ����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ��ɴ˹����Ƴ���Ӧ��12hʱ��Ӧ���Ũ��c��A��Ϊ_______mol��L-1
��1�������¶ȡ�����ѹǿ
��2����(A)="("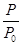 ��1)100%��94.1%��
��1)100%��94.1%��
��3����0.1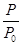 ��0.1��2��
��0.1��2��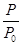 ����
����
��0.051��1�֣���ÿ���4Сʱ��A��Ũ��Ϊԭ����һ�롣0.013��1�֣�
��2����(A)="("
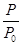 ��1)100%��94.1%��
��1)100%��94.1%����3����0.1
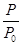 ��0.1��2��
��0.1��2��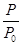 ����
������0.051��1�֣���ÿ���4Сʱ��A��Ũ��Ϊԭ����һ�롣0.013��1�֣�
�����������1�� �����A��ƽ��ת���ʼ��ô˷�Ӧ������Ӧ�����ƶ����˷�Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ�����������ƶ����˷�Ӧ���������������ķ�Ӧ������ѹǿ��ƽ������������ٵķ����������ƶ���
��2�� ����ͬ�����£�ѹǿ֮�ȵ������ʵ���֮�ȡ��ڴ˷�Ӧ�� A(g)
 B(g)��C(g)��P/P0=n/n0��n��n0�ֱ�Ϊ���������ʵ�������ʼʱ��������ʵ������ڷ�Ӧ�����ӵ����ʵ����ͷ�Ӧ��A�����ʵ�����ȣ�����A��ת����Ϊ(n-n0)/n0100%=" (P-" P0)/P0100%="("
B(g)��C(g)��P/P0=n/n0��n��n0�ֱ�Ϊ���������ʵ�������ʼʱ��������ʵ������ڷ�Ӧ�����ӵ����ʵ����ͷ�Ӧ��A�����ʵ�����ȣ�����A��ת����Ϊ(n-n0)/n0100%=" (P-" P0)/P0100%="(" 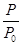 ��1)100%��ƽ��ʱA��ת����Ϊ��9.53-4.91��/4.91
��1)100%��ƽ��ʱA��ת����Ϊ��9.53-4.91��/4.91=94.1%��
����ʼʱ��1.0 L�ܱ������з���0.10molA(g)��n�� =" n(A)+" n(A)����(A)=" n(A)��(1+��(A))=" 0.1
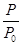 mol��n(A)= 0.1��2��
mol��n(A)= 0.1��2��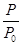 ��mol��
��mol���ڶ��շ�Ӧʱ��ͷ�Ӧ��A��Ũ�ȵĹ�ϵ���ɵõ���

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
�����Ŀ
 CH3OH(g)���������������������£��о��¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ������˵����ȷ����
CH3OH(g)���������������������£��о��¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ������˵����ȷ����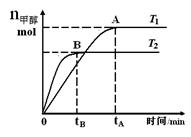
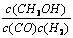
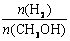 ����
����
 CH4 (g)+2H2O(g)
CH4 (g)+2H2O(g)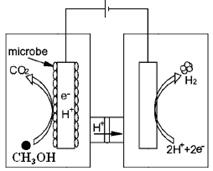

 Sn2��(aq)��Pb(s)����ϵ��c(Pb2��)��c(Sn2��)�仯��ϵ����ͼ��ʾ�������ж���ȷ����
Sn2��(aq)��Pb(s)����ϵ��c(Pb2��)��c(Sn2��)�仯��ϵ����ͼ��ʾ�������ж���ȷ����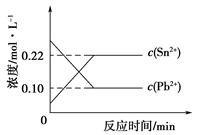
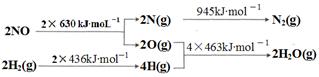
 2NO���ǵ�������β���к���NO��ԭ��֮һ��
2NO���ǵ�������β���к���NO��ԭ��֮һ��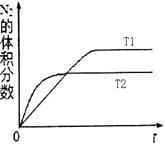
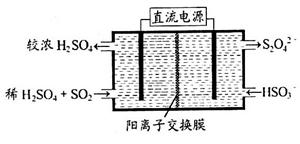
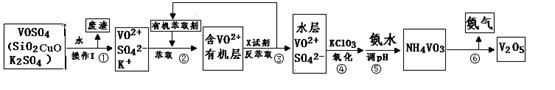
 VOM2���л��㣩 + H2SO4 (ˮ��)
VOM2���л��㣩 + H2SO4 (ˮ��)