��Ŀ����
����Ŀ��(1)CH3CH2CH(C2H5)CH(CH3)2��ϵͳ����Ϊ______��
(2)����ʽΪC4H8����֧����ϩ����һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ______������ϩ��ͨ��������Ȼ�̼��Һ�У������ķ�Ӧ����Ϊ______��
(3)�Ҵ����������������ŵ�����Ϊ_____������ʽΪ_____����д�����Ҵ���Է���������ȵ�������Ҵ�����������Ӧ�Ļ�ѧ����ʽ______��
(4)��֪����A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ.��AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
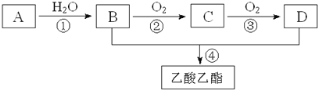
��A�й����ŵ�����Ϊ_______
�ڷ�Ӧ�ڵĻ�ѧ����ʽ_____��
���𰸡�2-��-3-�һ����� 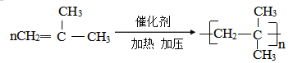 �ӳɷ�Ӧ �ǻ�
�ӳɷ�Ӧ �ǻ� ![]() HCOOH+C2H5OH
HCOOH+C2H5OH![]() HCOOC2H5+H2O ̼̼˫�� 2CH3CH2OH+O2
HCOOC2H5+H2O ̼̼˫�� 2CH3CH2OH+O2![]() 2CH3CHO+2H2O
2CH3CHO+2H2O
��������
(1)����ϵͳ����������ѡ�̼��Ϊ������ȡ������λ��֮����С���ɵ�CH3CH2CH(C2H5)CH(CH3)2��ϵͳ����Ϊ��2-��-3-�һ����飻
(2) ����ʽΪC4H8�Ҵ���֧����ϩ��ֻ��һ�ֽṹ�����䷢���Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��
(3)�Ҵ��ķ���ʽΪ��C2H5OH�����������ŵ�����Ϊ�ǻ����ǻ��ĵ���ʽΪ��![]() �����Ҵ���Է���������ȵ������Ǽ��ᣬ�������Ҵ�����������Ӧ�Ļ�ѧ����ʽΪ��HCOOH+C2H5OH
�����Ҵ���Է���������ȵ������Ǽ��ᣬ�������Ҵ�����������Ӧ�Ļ�ѧ����ʽΪ��HCOOH+C2H5OH![]() HCOOC2H5+H2O��
HCOOC2H5+H2O��
(4)��������ͼ����Ϣ�����ƶϣ�A����ϩ��B���Ҵ���C����ȩ��D�����ᣬ�ݴ˽�𣻢�A�й����ŵ������ǣ�̼̼˫������Ӧ�����Ҵ���������ȩ�ķ�Ӧ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���ڼס��ҡ���������ͬ�ܱ������а���ͬ��ʽͶ�ϣ�һ�������·�����Ӧ����ʼ�¶Ⱥ���ʼ�����ͬ����A2(g)+2B2(g)![]() 2AB3(g) ��H��0������������±���ʾ��
2AB3(g) ��H��0������������±���ʾ��
���� | �� | �� | �� |
������� | ���º��� | ���Ⱥ��� | ���º�ѹ |
��Ӧ��Ͷ�� | 1mol A2��2molB2 | 2molAB3 | 2mol AB3 |
��Ӧ���ת���� | a�� | a�� | a�� |
��Ӧ��ƽ�ⳣ��K= | K�� | K�� | K�� |
ƽ��ʱAB3��Ũ��/mol��L-1 | c�� | c�� | c�� |
ƽ��ʱAB3�ķ�Ӧ����/mol��L-1��min-1 | v�� | v�� | v�� |
����˵����ȷ����( )
A.v��=v��B.c����c��C.a�� +a����1D.K��
����Ŀ����X��Y��Z��W���ֺ�14�����ӵ����ӣ���ṹ�ص����£�
���Ӵ��� | X | Y | Z | W |
ԭ�Ӻ��� | ���� | ��ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� |
���ӵĵ���� | 0 | 0 | ��������� | 0 |
![]() ԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ______ ��
ԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ______ ��![]() �������ᄃ���к��й��ۼ���ĿΪ ______
�������ᄃ���к��й��ۼ���ĿΪ ______
![]() ���������ɵĻ�����ĵ���ʽΪ ______
���������ɵĻ�����ĵ���ʽΪ ______
![]() ��ȫȼ�շų���������
��ȫȼ�շų���������![]() ��д��Yȼ�յ��Ȼ�ѧ����ʽ ______
��д��Yȼ�յ��Ȼ�ѧ����ʽ ______
![]() ���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ
���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ![]() ��Ӧ������������������
��Ӧ������������������![]()
![]()
![]() д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ______
д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ______
![]() ���W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� ______ �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ______ ��
���W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� ______ �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ______ ��