��Ŀ����
����Ŀ��ij�о���ѧϰС������������о�����ˮ��Ӧ���ù������ʵijɷ֡����ʼ���������ʵ��̽��������ͬ����������⣺

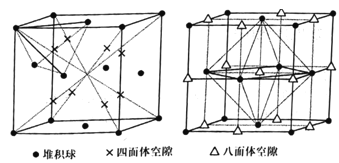
̽��һ�������ͼ��ʾװ�ý���������ˮ��Ӧ����ʵ��
��1��Ӳ�ʲ�����E�з�Ӧ�Ļ�ѧ����ʽΪ_________
��2����ӦǰA��Ͷ�����Ƭ��Ŀ����_________
��3��װ��E�е�������_________
̽�������������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ������к�ɫ����ijɷ֡�
ʵ���������Ӳ�ʲ�����B��ȴ��ȡ�������еĹ�����������ϡ�����������Һ�ֳ����ݡ�
��4��һ�ݵμӼ���KSCN��Һ������Һ��Ѫ��ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ________��ѡ����ţ���ͬ����д����Һ��Ѫ��ɫ�����ӷ���ʽ��____________������Һδ��Ѫ��ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ_________��
��һ����Fe3O4 ��һ����Fe ��ֻ��Fe3O4 ��ֻ��Fe
��5����һ����_________�����������ƣ�������_________�����Լ������۲�۲쵽_________����ʵ����������֤����Һ�д���Fe2+��
���𰸡�H2+CuO![]() Cu+H2O ��ֹ���� ��ɫ�����죬�Ҷ˹ܱ���ˮ�� �� Fe3++3SCN-=Fe��SCN��3 �� ��ͷ�ι� ����KMnO4��Һ ��Һ��ɫ��ȥ
Cu+H2O ��ֹ���� ��ɫ�����죬�Ҷ˹ܱ���ˮ�� �� Fe3++3SCN-=Fe��SCN��3 �� ��ͷ�ι� ����KMnO4��Һ ��Һ��ɫ��ȥ
��������
ʵ��A����ȡˮ������B��Fe��ˮ������Ӧ����������������������C�ռ�������D���ڸ���������E��������ԭCuO��֤�����������ɣ�
A����ȡˮ������B��Fe��ˮ������Ӧ����������������������C�ռ�������D���ڸ���������E��������ԭCuO��
��1��Ӳ�ʲ�����E�з�Ӧ�Ļ�ѧ����ʽΪH2+CuO![]() Cu+H2O��
Cu+H2O��
�𰸣�H2+CuO![]() Cu+H2O
Cu+H2O
��2�����Ƭ�з��������ã���ֹ������ȫ�¹ʣ�
�𰸣���ֹ���У�
��3��CuO�ʺ�ɫ��Cu�ʺ�ɫ��������ԭCuO�õ�Cu����ͬʱ����ˮ�����������Ǻ�ɫ�����죬�Ҷ˹ܱ���ˮ�飻
�𰸣���ɫ�����죬�Ҷ˹ܱ���ˮ��
��4�������Ӻ�KSCN��Ӧ����������ʹ��Һ��Ѫ��ɫ���������Ӻ�KSCN����Ӧ������Һ�м���KSCN��Һ�ʺ�ɫ��˵���������������������ܺ���Fe�������Ӻ�Fe��Ӧ�����������ӣ������ɫ˵�������к���������������Fe��
�𰸣� �� Fe3++3SCN-=Fe��SCN��3 ��
��5���������Ӿ��л�ԭ�ԣ��ܱ����Ը��������Һ���������Կ��������Ը��������Һ�����������ӣ�
�𰸣���ͷ�ι� ����KMnO4��Һ ��Һ��ɫ��ȥ

����Ŀ��ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̡�
(1)��ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪ��
C(s)+H2O(g)![]() CO(g)+H2(g)����H= +131.3kJ/mol����S= +133.7J/(K��mol)��
CO(g)+H2(g)����H= +131.3kJ/mol����S= +133.7J/(K��mol)��
���÷�Ӧ�ܷ��Է�������________________�йء�
��һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����________(����ĸ����ͬ)��
a�������е�ѹǿ����
b��1mol H-H�����ѵ�ͬʱ����2mol H-O��
c��v��(CO)��v��(H2O)
d��c(CO)=c(H2)
(2)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO(g)+H2O(g)![]() CO2(g)+H2(g)���õ������������ݣ�
CO2(g)+H2(g)���õ������������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
H2O | CO | H2 | CO | |||
1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
3 | 900 | a | b | c | d | t |
��ʵ��1����v(CO2)��ʾ�ķ�Ӧ����Ϊ__________��
���÷�Ӧ���淴ӦΪ________(����������������)�ȷ�Ӧ��
����ʵ��3Ҫ�ﵽ��ʵ��2��ͬ��ƽ��״̬(�������ʵ����������ֱ����)����t<3min����a��bӦ����Ĺ�ϵ��____________________(�ú�a��b����ѧʽ��ʾ)��
(3)Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ/mol)�ı仯�������Ϊ1L�ĺ����ܱ������У�����1mol CO2��3mol H2�����д�ʩ����ʹ c(CH3OH)�������___��
CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ/mol)�ı仯�������Ϊ1L�ĺ����ܱ������У�����1mol CO2��3mol H2�����д�ʩ����ʹ c(CH3OH)�������___��

a�������¶� b������He(g)��ʹ��ϵѹǿ����
c����H2O(g)����ϵ�з������ ����d���ٳ���1mol CO2��3mol H2
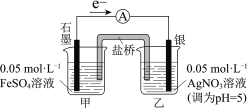
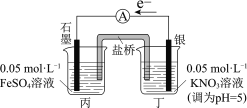
����Ŀ��ijͬѧ�о�FeSO4��Һ��AgNO3��Һ�ķ�Ӧ��������¶Ա�ʵ�顣
ʵ�� |
�� |
�� |
���� | ��ͨ��·������ָ������ƫת���ֱ�ȡ��Ӧǰ�ͷ�Ӧһ��ʱ�����ձ��е���Һ���μ�KSCN��Һ��ǰ������ɫ,�����Ժ�ɫ | ��ͨ��·������ָ��������С��ƫת���������ձ��о����������� |
����˵����ȷ����
A.���ɢ��е��������֪Ag+��������ǿ��Fe3+
B.���е�����ָ������ƫת��ԭ����Fe2+���������缫
C.�����������缫����ʯī�缫��������ָ����ܲ�������ƫת
D.�ԱȢ��֪������NO3��������Fe2+