��Ŀ����
ij���᳧������Ϊ�˷�ֹ������Ⱦ����β�������ۺ����ã��������ַ�������β���е�SO2�����壮��1������һ����β��ͨ�백ˮ����������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���壮Ϊ�ⶨ���ɵģ�NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ�û������Ʒ4�ݣ��ֱ�����Ũ�ȵ�NaOH��Һ50.00mL��ʹ���ɵİ���ȫ���ݳ�������й�ʵ���������£�
| ʵ�� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L����״���� |
| 1 | 7.24 | 50.00 | 1.792 |
| 2 | 14.48 | 50.00 | 3.584 |
| 3 | 21.72 | 50.00 | 4.032 |
| 4 | 36.20 | 50.00 | 2.240 |
��2������������NaOH��Һ��ʯ�Ҽ�O2����β��SO2������ȡʯ�ࣨCaSO4?2H20�����˹��̵��м������NaHSO3������β���ŷŵ������ɻ��SO2��NaOH���ʵ�������ѱȣ��Ӷ�������������ƵIJ�������ͼ��ʾn��NaHSO3�� ��n��SO2��/n��NaOH����ֵͬʱ�ı仯���ߣ�����д���пհף�
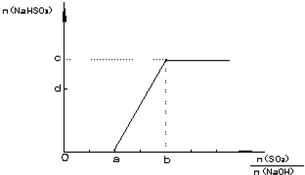
��a=
��c=
��������1����H++OH-=H2O��NH4++OH-=NH3��+H2O������֪����Ʒ���������Ʒ�Ӧ�����ݷ�����֪1��2ʵ�����������ƹ�����������ݼ�������狀�����������ʵ����õ���NH4��2SO4��NH4HSO4�����������ɱȣ�����3��4 ʵ�������֪�������Ʋ��㣬�������ã�NH4��2SO4��NH4HSO4�����������ɱȼ���õ����������������ʵ�������õ�Ũ�ȣ�
��2�����ݷ�ӦSO2+2NaOH=Na2SO3+H2O��SO2+NaOH=NaHSO3��������ϵ��������n��SO2����n��NaOH��=1��2�����������ƣ�n��SO2����n��NaOH��=1��1��Ӧ�������������ƣ�����ͼ������ϵ���㣬c���������������������ʱ���ʵ�����d�����������Ʋ��ַ�Ӧ�������������ƣ�
��2�����ݷ�ӦSO2+2NaOH=Na2SO3+H2O��SO2+NaOH=NaHSO3��������ϵ��������n��SO2����n��NaOH��=1��2�����������ƣ�n��SO2����n��NaOH��=1��1��Ӧ�������������ƣ�����ͼ������ϵ���㣬c���������������������ʱ���ʵ�����d�����������Ʋ��ַ�Ӧ�������������ƣ�
����⣺��1����H++OH-=H2O NH4++OH-=NH3��+H2O
��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊx��y���ɵ�1����2�������ݣ�NaOH��������
�ã�115x+132y=7.24
x+2y=1.792��22.4
x=0.04mol
y=0.02mol
�ɵ�4����3�������ݣ�NaOH�����㣬��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊa��b
��a��b=2��1 ��
=
a=0.2mol
c��NaOH��=
=6mol/L
�ʴ�Ϊ����H++OH-=H2O NH4++OH-=NH3��+H2O
��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊx��y���ɵ�1����2�������ݣ�NaOH��������
�ã�115x+132y=7.24
x+2y=1.792��22.4
x=0.04mol
y=0.02mol
�ɵ�4����3�������ݣ�NaOH�����㣬��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊa��b
��a��b=2��1 ��
=
a=0.2mol
c��NaOH��=
=6mol/L��
��2����SO2+2NaOH=Na2SO3+H2O��SO2+NaOH=NaHSO3��������ϵ��������n��SO2����n��NaOH��=1��2�����������ƣ�n��SO2����n��NaOH��=1��1��Ӧ�������������ƣ�����ͼ����a=0.5��b=1��
�ʴ�Ϊ��0.5��1.0��
��ͼ�������֪c��Ϊ������������������ƣ����շ�ӦSO2+NaOH=NaHSO3������c=n��NaOH����d����̼���Ʋ��ֱ仯Ϊ̼�����ƣ����ݻ�ѧ����ʽ����õ���
SO2+2NaOH=Na2SO3+H2O
1 2
n��NaOH�� n��NaOH��
SO2+H2O+Na2SO3=2NaHSO3��
1 2
n��SO2��-
n��NaOH�� 2n��SO2��-n��NaOH��
d=2n��SO2��-n��NaOH��
�ʴ�Ϊ��n��NaOH����2n��SO2��-n��NaOH����
��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊx��y���ɵ�1����2�������ݣ�NaOH��������
�ã�115x+132y=7.24
x+2y=1.792��22.4
x=0.04mol
y=0.02mol
�ɵ�4����3�������ݣ�NaOH�����㣬��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊa��b
��a��b=2��1 ��
| a | ||
36.2-
|
| 2 |
| 1 |
c��NaOH��=
(0.2+
| ||
| 0.05L |
�ʴ�Ϊ����H++OH-=H2O NH4++OH-=NH3��+H2O
��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊx��y���ɵ�1����2�������ݣ�NaOH��������
�ã�115x+132y=7.24
x+2y=1.792��22.4
x=0.04mol
y=0.02mol
�ɵ�4����3�������ݣ�NaOH�����㣬��NH4HSO4�� ��NH4��2SO4���ʵ����ֱ�Ϊa��b
��a��b=2��1 ��
| a | ||
36.2-
|
| 2 |
| 1 |
c��NaOH��=
(0.2+
| ||
| 0.05L |
��2����SO2+2NaOH=Na2SO3+H2O��SO2+NaOH=NaHSO3��������ϵ��������n��SO2����n��NaOH��=1��2�����������ƣ�n��SO2����n��NaOH��=1��1��Ӧ�������������ƣ�����ͼ����a=0.5��b=1��
�ʴ�Ϊ��0.5��1.0��
��ͼ�������֪c��Ϊ������������������ƣ����շ�ӦSO2+NaOH=NaHSO3������c=n��NaOH����d����̼���Ʋ��ֱ仯Ϊ̼�����ƣ����ݻ�ѧ����ʽ����õ���
SO2+2NaOH=Na2SO3+H2O
1 2
| 1 |
| 2 |
SO2+H2O+Na2SO3=2NaHSO3��
1 2
n��SO2��-
| 1 |
| 2 |
d=2n��SO2��-n��NaOH��
�ʴ�Ϊ��n��NaOH����2n��SO2��-n��NaOH����
���������⿼����������ɵļ��������ͼ������жϵķ����Ͷ�������Ӧ�ã���ѧ����ʽ���йؼ��㣬���ջ��������������ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
�Ӵ����������ŷŵ�β���У��������Ķ�������ij���᳧Ϊ�˷�ֹ������Ⱦ�����跨��β�������ۺ����ã����ð�ˮ����β���еĶ���������������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���塣Ϊ�ⶨ������NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00 mL��������120 �����ң�ʹ����ȫ���ݳ�����NH4��2SO4��NH4HSO4�ֽ��¶Ⱦ�����200 �桳������й�ʵ���������£���״������
ʵ����� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L |
�� | 7.4 | 40.00 | 1.68 |
�� | 14.8 | 40.00 | 3.36 |
�� | 22.2 | 40.00 | 1.12 |
�� | 37.0 | 40.00 | 0.00 |
��1��ʵ��������йط�Ӧ�����ӷ���ʽΪ��________________________________��
��2���ɢ�������ֱ���Ʋ⣺��״������3.7 g��Ʒ����ͬ��ʵ��ʱ�����ɰ��������Ϊ________L��
��3���Լ���û�����еģ�NH4��2SO4��NH4HSO4���ʵ���֮��________��
��4���������NaOH��Һ�����ʵ���Ũ�ȣ�Ӧѡ���________�����ݣ��ɴ����NaOH��Һ�����ʵ���Ũ��Ϊ________��
 ����ֹ��Ⱦ�������������ԭ�ϣ����ŷ�ǰ�������β���������跨�����ۺ����á�
����ֹ��Ⱦ�������������ԭ�ϣ����ŷ�ǰ�������β���������跨�����ۺ����á� ��Һ���գ�Ȼ���ټ���������Һ��д�����������еĻ�ѧ��Ӧ����ʽ��
�����·����봫ͳ������ȣ����ŵ��� ��
��Һ���գ�Ȼ���ټ���������Һ��д�����������еĻ�ѧ��Ӧ����ʽ��
�����·����봫ͳ������ȣ����ŵ��� �� ( O
( O